J Korean Ophthalmol Soc.
2018 May;59(5):403-409. 10.3341/jkos.2018.59.5.403.
The Effect of 5% Serum Albumin on Intractable Corneal Epithelial Keratitis: a Case Series and Literature Review
- Affiliations
-
- 1Department of Ophthalmology and Visual Science, College of Medicine, The Catholic University of Korea, Seoul, Korea. yangkyeung@hanmail.net
- KMID: 2411448
- DOI: http://doi.org/10.3341/jkos.2018.59.5.403
Abstract
- PURPOSE
We report five cases of intractable epithelial keratitis in which 5% serum albumin showed significant effects as a topical treatment when compared with other previous conservative treatments based on a literature review.
METHODS
This was a retrospective observational case series of five patients with intractable corneal epithelial keratitis who used serum albumin as a topical treatment. All patients had already been using other conservative treatments such as non-preserved artificial tears and autologous serum eye drops. The size of the epithelial defect and the time taken for closure of the epithelial defect were observed.
RESULTS
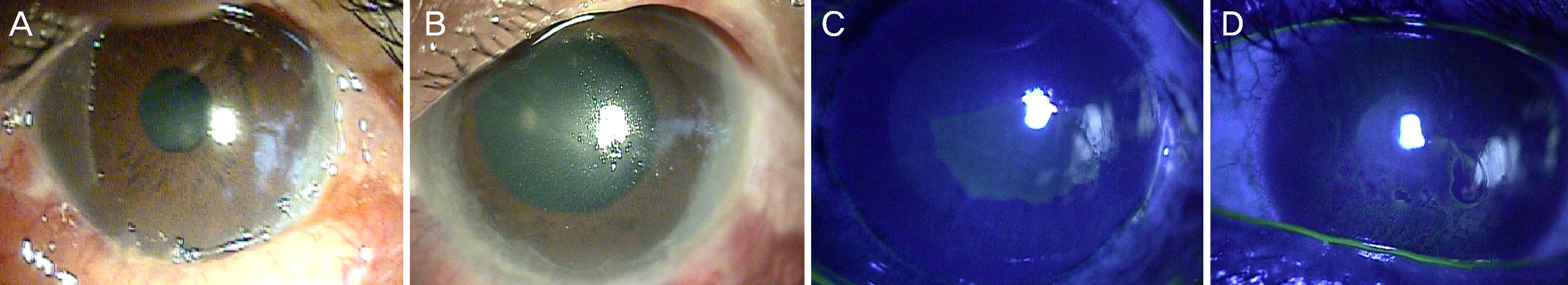
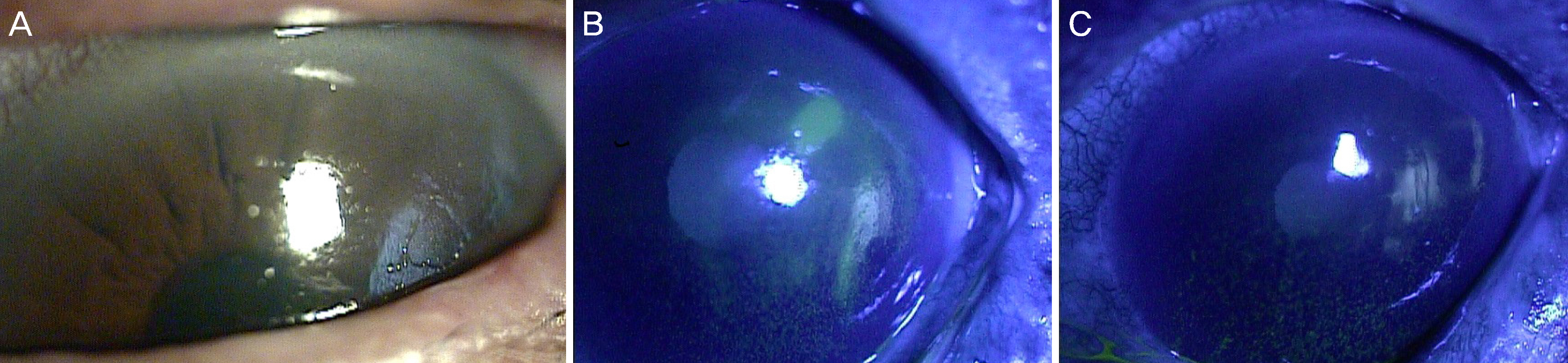
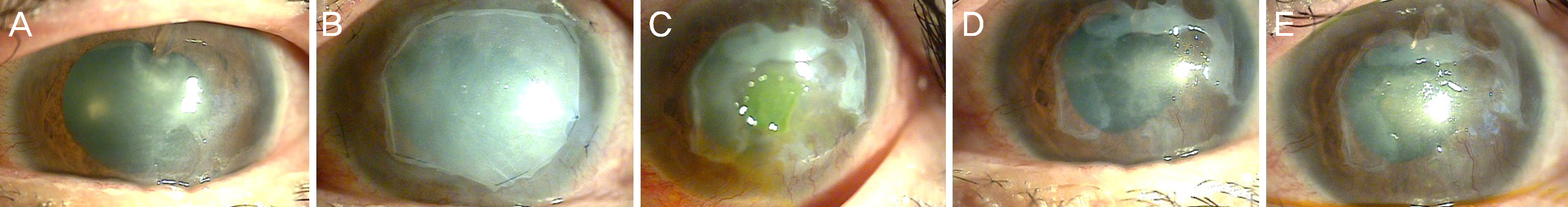
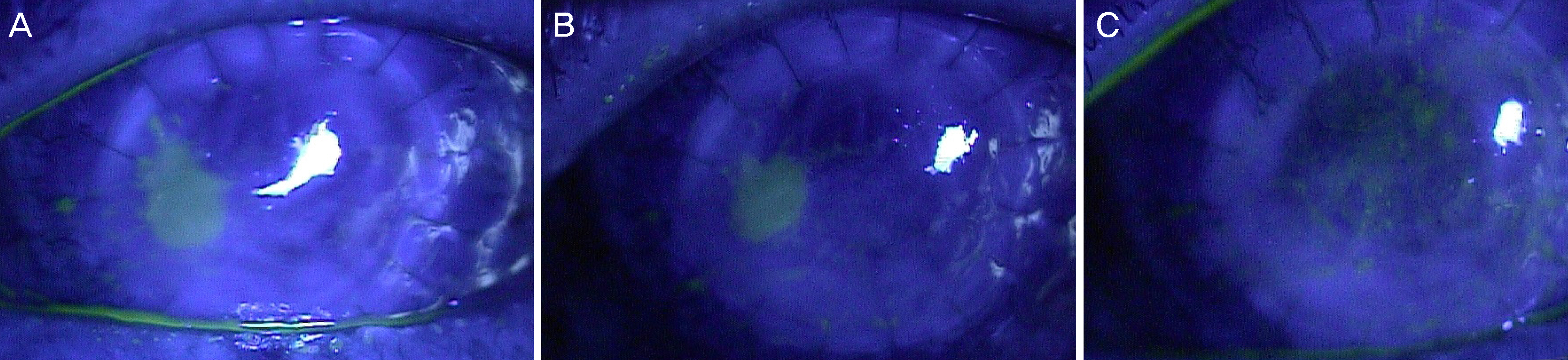
The study involved five patients with intractable corneal epithelial disease with a mean follow-up time of 6 months. The epithelial lesions improved with the use of 5% serum albumin from the beginning of treatment and lasted throughout the follow- up period. The mean time to complete epithelial closure using 5% serum albumin eye drops was 21 days.
CONCLUSIONS
Treatment with 5% serum albumin eye drops may have a significant and stable effect in the treatment of intractable corneal epithelial disease.
MeSH Terms
Figure
Reference
-
References
1. Tsubota K, Higuchi A. Serum application for the treatment of abdominal surface disorders. Int Ophthalmol Clin. 2000; 40:113–22.2. Fukuda M, Wang HF. Dry eye and closed eye tears. Cornea. 2000; 19(3 Suppl):S44–8.
Article3. Geerling G, Unterlauft JD, Kasper K, et al. Autologous serum and alternative blood products for the treatment of ocular surface disorders. Ophthalmologe. 2008; 105:623–31.4. Seki JT, Sakurai N, Moldenhauer S, et al. Human albumin eye drops as a therapeutic option for the management of abdominal sicca secondary to chronic graft-versus-host disease after stem-cell allografting. Curr Oncol. 2015; 22:e357–63.5. del Castillo JM, de la Casa JM, Sardiña RC, et al. Treatment of abdominal corneal erosions using autologous serum. Cornea. 2002; 21:781–3.6. Ohashi Y, Motokura M, Kinoshita Y, et al. Presence of epidermal growth factor in human tears. Invest Ophthalmol Vis Sci. 1989; 30:1879–82.7. Ubels JL, Foley KM, Rismondo V. Retinol secretion by the abdominal gland. Invest Ophthalmol Vis Sci. 1986; 27:1261–8.8. Fox RI, Chan R, Michelson JB, et al. Beneficial effect of artificial tears made with autologous serum in patients with abdominal sicca. Arthritis Rheum. 1984; 27:459–61.9. Tananuvat N, Daniell M, Sullivan LJ, et al. Controlled study of the use of autologous serum in dry eye patients. Cornea. 2001; 20:802–6.
Article10. Ogawa Y, Okamoto S, Mori T, et al. Autologous serum eye drops for the treatment of severe dry eye in patients with chronic graftversus-host disease. Bone Marrow Transplant. 2003; 31:579–83.
Article11. Noble BA, Loh RS, MacLennan S, et al. Comparison of autologous serum eye drops with conventional therapy in a randomised abdominalled crossover trial for ocular surface disease. Br J Ophthalmol. 2004; 88:647–52.12. Poon AC, Geerling G, Dart JK, et al. Autologous serum eyedrops for dry eyes and epithelial defects: clinical and in vitro toxicity studies. Br J Ophthalmol. 2001; 85:1188–97.
Article13. Tsubota K, Goto E, Fujita H, et al. Treatment of dry eye by abdominal serum application in Sjögren's syndrome. Br J Ophthalmol. 1999; 83:390–5.14. Matsumoto Y, Dogru M, Goto E, et al. Autologous serum application in the treatment of neurotrophic keratopathy. Ophthalmology. 2004; 111:1115–20.15. Tsubota K, Goto E, Shimmura S, Shimazaki J. Treatment of persistent corneal epithelial defect by autologous serum application. Ophthalmology. 1999; 106:1984–9.
Article16. Han MS, Lee JH, Lee SJ. Therapeutic effect of topical autologous serum in recurrent punctate corneal erosion. J Korean Ophthalmol Soc. 2004; 45:1639–44.17. Choi JH, Oh HJ, Yoon KC. Effect of combined treatment with abdominal A and cord serum for dry eye associated with graft-abdominal-host-disease. J Korean Ophthalmol Soc. 2013; 54:587–94.18. Lee GA, Chen SX. Autologous serum in the management of abdominal dry eye syndrome. Clin Exp Ophthalmol. 2008; 36:119–22.19. Eberle J, Habermann J, Gürtler LG. HIV-1 infection transmitted by serum droplets into the eye: a case report. AIDS. 2000; 14:206–7.
Article20. Weisbach V, Dietrich T, Kruse FE, et al. HIV and hepatitis B/C abdominals in patients donating blood for use as autologous serum eye drops. Br J Ophthalmol. 2007; 91:1724–5.21. Ralph RA, Doane MG, Dohlman CH. Clinical experience with a mobile ocular perfusion pump. Arch Ophthalmol. 1975; 93:1039–43.
Article22. Coyle PK, Sibony PA, Johnson C. Electrophoresis combined with immunologic identification of human tear proteins. Invest Ophthalmol Vis Sci. 1989; 30:1872–8.23. Kuizenga A, van Haeringen NJ, Kijlstra A. SDS-Minigel abdominal of human tears. Effect of sample treatment on protein patterns. Invest Ophthalmol Vis Sci. 1991; 32:381–6.24. Nicholson JP, Wolmarans MR, Park GR. The role of albumin in critical illness. Br J Anaesth. 2000; 85:599–610.
Article25. Higuchi A, Ueno R, Shimmura S, et al. Albumin rescues ocular abdominal cells from cell death in dry eye. Curr Eye Res. 2007; 32:83–8.26. Shimmura S, Ueno R, Matsumoto Y, et al. Albumin as a tear abdominal in the treatment of severe dry eye. Br J Ophthalmol. 2003; 87:1279–83.27. Cao G, Alessio HM, Cutler RG. Oxygen-radical absorbance abdominal assay for antioxidants. Free Radic Biol Med. 1993; 14:303–11.28. Cantin AM, Paquette B, Richter M, Larivée P. Albumin-mediated regulation of cellular glutathione and nuclear factor kappa B activation. Am J Respir Crit Care Med. 2000; 162:1539–46.29. Zhang WJ, Frei B. Albumin selectively inhibits TNF alpha-abdominal expression of vascular cell adhesion molecule-1 in human aortic endothelial cells. Cardiovasc Res. 2002; 55:820–9.30. Unterlauft JD, Kohlhaas M, Hofbauer I, et al. Albumin eye drops for treatment of ocular surface diseases. Ophthalmologe. 2009; 106:932–7.31. Schargus M, Kohlhaas M, Unterlauft JD. Treatment of severe abdominal surface disorders with albumin eye drops. J Ocul Pharmacol Ther. 2015; 31:291–5.32. Petrides PE, Hintz RL, Bohlen P, Shively JE. An improved method for the purification of human insulin-like growth factors I and II. Endocrinology. 1986; 118:2034–8.
Article33. Kojima T, Higuchi A, Goto E, et al. Autologous serum eye drops for the treatment of dry eye diseases. Cornea. 2008; 27(Suppl 1):S25–30.
Article34. Yu FS, Yin J, Xu K, Huang J. Growth factors and corneal epithelial wound healing. Brain Res Bull. 2010; 81:229–35.
Article35. Gupta A, Monroy D, Ji Z, et al. Transforming growth factor beta-1 and beta-2 in human tear fluid. Curr Eye Res. 1996; 15:605–14.
Article36. Cho YK, Huang W, Kim GY, Lim BS. Comparison of autologous serum eye drops with different diluents. Curr Eye Res. 2013; 38:9–17.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dendritic Keratitis Associated with Contact Lens Wear: a Case Series and Literature Review
- Ultrastructural Changes of Corneal Epithelium and Basement Membrane in Neurotrophic Corneal Ulcer
- Cases of Amniotic Membrane Transplantation for Herpetic Keratitis
- Management of Fungal Ocular Infection with Topical and Intracameral Voriconazole
- A Case of Congenital Syphilitic Interstitial Keratitis