Cancer Res Treat.
2018 Apr;50(2):506-517. 10.4143/crt.2016.607.
Redefining the Positive Circumferential Resection Margin by Incorporating Preoperative Chemoradiotherapy Treatment Response in Locally Advanced Rectal Cancer: A Multicenter Validation Study
- Affiliations
-
- 1Department of Radiation Oncology, Seoul National University College of Medicine, Seoul, Korea. ekchie93@snu.ac.kr
- 2Department of Surgery, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 4Center for Colorectal Cancer, Research Institute and Hospital, National Cancer Center, Goyang, Korea.
- 5Department of Radiation Oncology, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea.
- 6Department of Radiation Oncology, Samsung Medical Center, Sung-kyunkwan University School of Medicine, Seoul, Korea.
- 7Department of Surgery, Seoul National University Bundang Hospital, Seongnam, Korea.
- 8Department of Radiation Oncology, Seoul National University Bundang Hospital, Seongnam, Korea.
- KMID: 2411139
- DOI: http://doi.org/10.4143/crt.2016.607
Abstract
- PURPOSE
This study was conducted to validate the prognostic influence of treatment response among patients with positive circumferential resection margin for locally advanced rectal cancer.
MATERIALS AND METHODS
Clinical data of 197 patientswith positive circumferential resection margin defined as ≤ 2 mm after preoperative chemoradiotherapy followed by total mesorectal excision between 2004 and 2009 were collected for this multicenter validation study. All patients underwent median 50.4 Gy radiation with concurrent fluoropyrimidine based chemotherapy. Treatment response was dichotomized to good response, including treatment response of grade 2 or 3, and poor response, including grade 0 or 1.
RESULTS
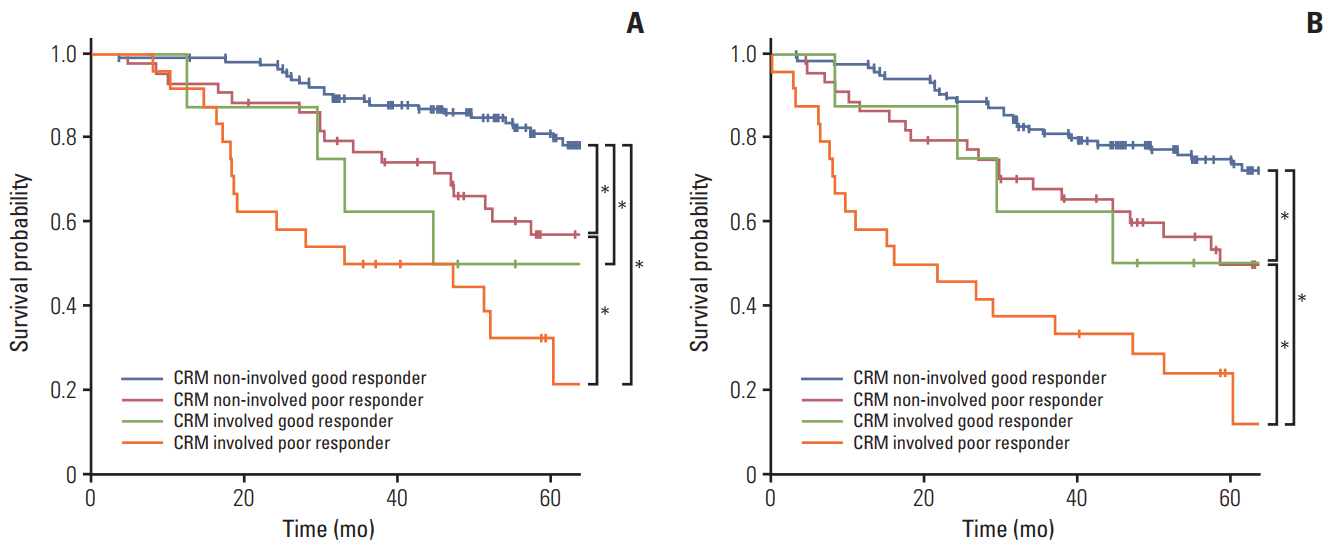
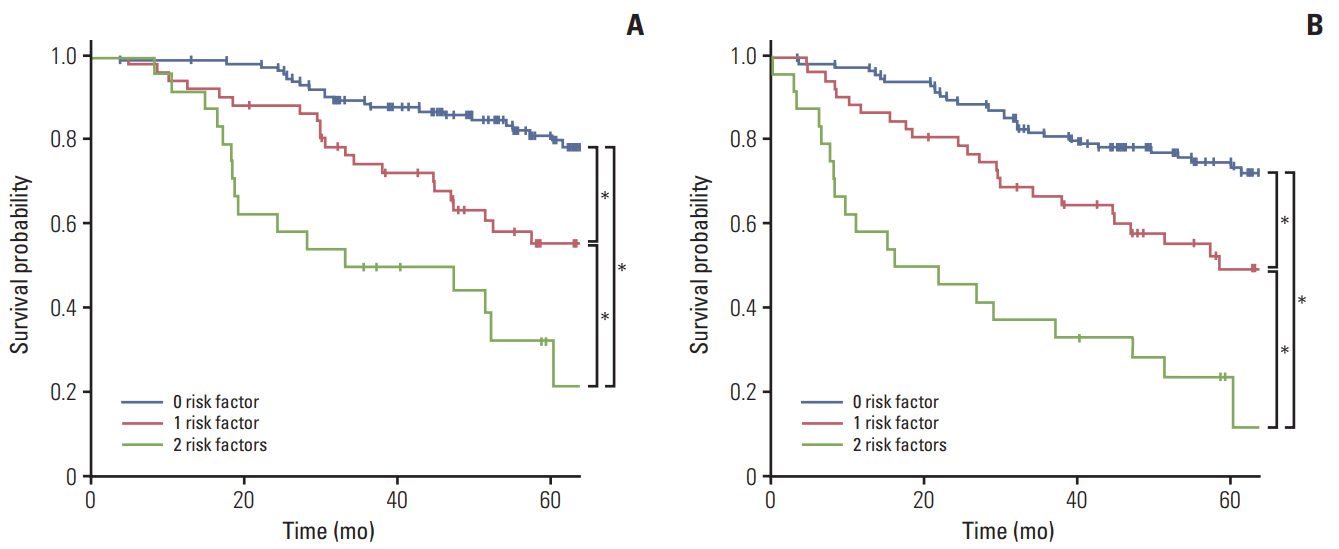
After 52 months median follow-up, 5-year overall survival (OS) for good responders and poor responders was 79.1% and 48.4%, respectively (p < 0.001). In multivariate analysis, circumferential resection margin involvement and treatment response were a prognosticator for OS and locoregional recurrence-free survival. In subgroup analysis, good responders with close margin showed significantly better survival outcomes for survival. Good responders with involved margin and poor responders with close margin shared similar results, whereas poor responders with involved margin had worst survival (5-year OS, 81.2%, 57.0%, 50.0%, and 32.4%, respectively; p < 0.001).
CONCLUSION
Among patients with positive circumferential resection margin after preoperative chemoradiotherapy, survival of the good responders was significantly better than poor responders. Subgroup analysis revealed that definition of positive circumferential resection margin may be individualized as involvement for good responders, whereas ≤ 2 mm for poor responders.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Adam IJ, Mohamdee MO, Martin IG, Scott N, Finan PJ, Johnston D, et al. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994; 344:707–11.
Article2. Quirke P, Durdey P, Dixon MF, Williams NS. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection: histopathological study of lateral tumour spread and surgical excision. Lancet. 1986; 2:996–9.3. Nagtegaal ID, Marijnen CA, Kranenbarg EK, van de Velde CJ, van Krieken JH; Pathology Review Committee, et al. Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit. Am J Surg Pathol. 2002; 26:350–7.4. Trakarnsanga A, Gonen M, Shia J, Goodman KA, Nash GM, Temple LK, et al. What is the significance of the circumferential margin in locally advanced rectal cancer after neoadjuvant chemoradiotherapy? Ann Surg Oncol. 2013; 20:1179–84.
Article5. Wibe A, Rendedal PR, Svensson E, Norstein J, Eide TJ, Myrvold HE, et al. Prognostic significance of the circumferential resection margin following total mesorectal excision for rectal cancer. Br J Surg. 2002; 89:327–34.
Article6. National Comprehensive Cancer Network. NCCN clincal practice guidelines in oncology: rectal cancer version 3.2014 [Internet]. Fort Washington, PA: National Comprehensive Cancer Network;2014. [cited 2014 Feb 4]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf.7. Lee JH, Chie EK, Kim K, Jeong SY, Park KJ, Park JG, et al. The influence of the treatment response on the impact of resection margin status after preoperative chemoradiotherapy in locally advanced rectal cancer. BMC Cancer. 2013; 13:576.
Article8. Bernstein TE, Endreseth BH, Romundstad P, Wibe A; Norwegian Colorectal Cancer Group. Circumferential resection margin as a prognostic factor in rectal cancer. Br J Surg. 2009; 96:1348–57.
Article9. Glynne-Jones R, Mawdsley S, Novell JR. The clinical significance of the circumferential resection margin following preoperative pelvic chemo-radiotherapy in rectal cancer: why we need a common language. Colorectal Dis. 2006; 8:800–7.
Article10. Rodel C, Martus P, Papadoupolos T, Fuzesi L, Klimpfinger M, Fietkau R, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005; 23:8688–96.11. Ruo L, Tickoo S, Klimstra DS, Minsky BD, Saltz L, Mazumdar M, et al. Long-term prognostic significance of extent of rectal cancer response to preoperative radiation and chemotherapy. Ann Surg. 2002; 236:75–81.
Article12. Bouzourene H, Bosman FT, Seelentag W, Matter M, Coucke P. Importance of tumor regression assessment in predicting the outcome in patients with locally advanced rectal carcinoma who are treated with preoperative radiotherapy. Cancer. 2002; 94:1121–30.
Article13. Edge SB, Byrd DR, Compton CC, Fritz AG, Greeene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer-Verlag;2010.14. Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997; 12:19–23.
Article15. Kim TH, Chang HJ, Kim DY, Jung KH, Hong YS, Kim SY, et al. Pathologic nodal classification is the most discriminating prognostic factor for disease-free survival in rectal cancer patients treated with preoperative chemoradiotherapy and curative resection. Int J Radiat Oncol Biol Phys. 2010; 77:1158–65.
Article16. Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009; 373:811–20.
Article17. Marijnen CA, Nagtegaal ID, Kapiteijn E, Kranenbarg EK, Noordijk EM, van Krieken JH, et al. Radiotherapy does not compensate for positive resection margins in rectal cancer patients: report of a multicenter randomized trial. Int J Radiat Oncol Biol Phys. 2003; 55:1311–20.
Article18. Alberda WJ, Verhoef C, Nuyttens JJ, van Meerten E, Rothbarth J, de Wilt JH, et al. Intraoperative radiation therapy reduces local recurrence rates in patients with microscopically involved circumferential resection margins after resection of locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2014; 88:1032–40.19. Gerard JP, Azria D, Gourgou-Bourgade S, Martel-Laffay I, Hennequin C, Etienne PL, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol. 2010; 28:1638–44.20. Rodel C, Liersch T, Becker H, Fietkau R, Hohenberger W, Hothorn T, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012; 13:679–87.21. Aschele C, Cionini L, Lonardi S, Pinto C, Cordio S, Rosati G, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011; 29:2773–80.
Article22. O'Connell MJ, Colangelo LH, Beart RW, Petrelli NJ, Allegra CJ, Sharif S, et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R-04. J Clin Oncol. 2014; 32:1927–34.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Surgical issues in locally advanced rectal cancer treated by preoperative chemoradiotherapy
- How Can We Improve the Tumor Response to Preoperative Chemoradiotherapy for Locally Advanced Rectal Cancer?
- Imaging Diagnosis of Locally Advanced Rectal Cancer: Tumor Staging before and after Preoperative Chemoradiotherapy
- The Effects and Surgical Morbidity of Preoperative Combined Chemoradiotherapy for Locally Advanced Rectal Cancer
- An Update on Preoperative Radiotherapy for Locally Advanced Rectal Cancer