Korean J Radiol.
2018 Jun;19(3):410-416. 10.3348/kjr.2018.19.3.410.
Paclitaxel-Coated Balloon Angioplasty for Early Restenosis of Central Veins in Hemodialysis Patients: A Single Center Initial Experience
- Affiliations
-
- 1Division of Interventional Radiology, Department of Radiology, Faculty of Medicine, Prince of Songkla University, Hat Yai 90110, Thailand. hkeerati@medicine.psu.ac.th
- 2Division of Nephrology, Department of Internal Medicine, Faculty of Medicine, Prince of Songkla University, Hat Yai 90110, Thailand.
- 3Division of Vascular Surgery, Department of Surgery, Faculty of Medicine, Prince of Songkla University, Hat Yai 90110, Thailand.
- KMID: 2410811
- DOI: http://doi.org/10.3348/kjr.2018.19.3.410
Abstract
OBJECTIVE
To report the results of angioplasty with paclitaxel-coated balloons for the treatment of early restenosis of central veins in hemodialysis patients.
MATERIALS AND METHODS
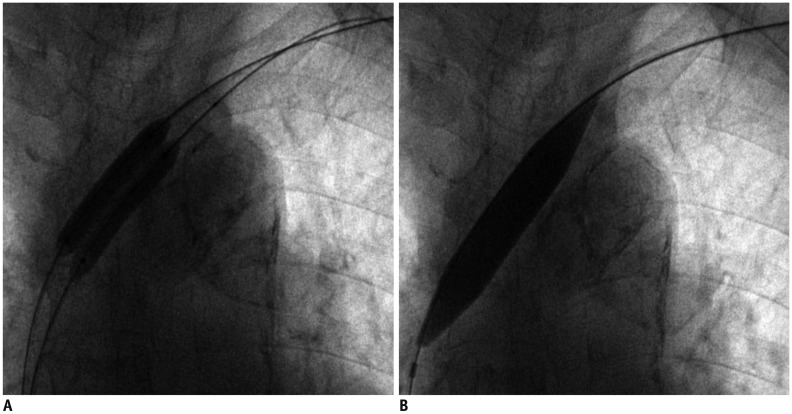
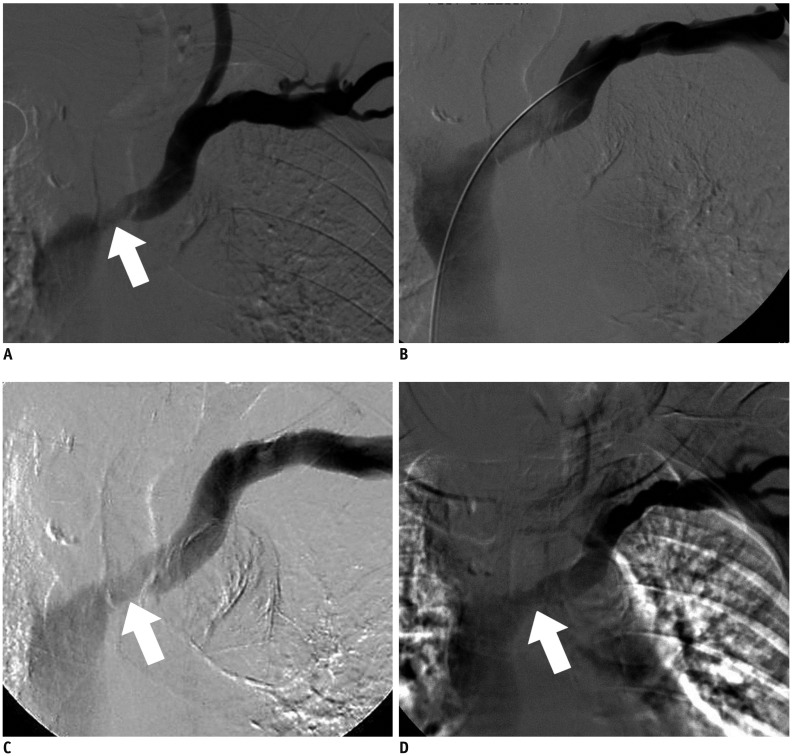
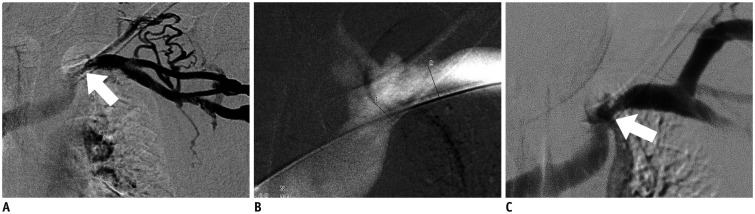
Sixteen patients (9 men and 7 women; mean age 65.8 ± 14.4 years; range, 40-82 years) with 16 episodes of early restenoses of central veins within 3 months (median patency duration 2.5 months) were enrolled from January 2014 to June 2015. Ten native central veins and 6 intra-stent central veins were treated with double paclitaxel-coated balloons (diameter 6-7 mm) plus a high pressure balloon (diameter 12-14 mm). The study outcomes included procedural success (< 30% residual stenosis) and primary patency of the treated lesion (< 50% angiographic stenosis without re-intervention).
RESULTS
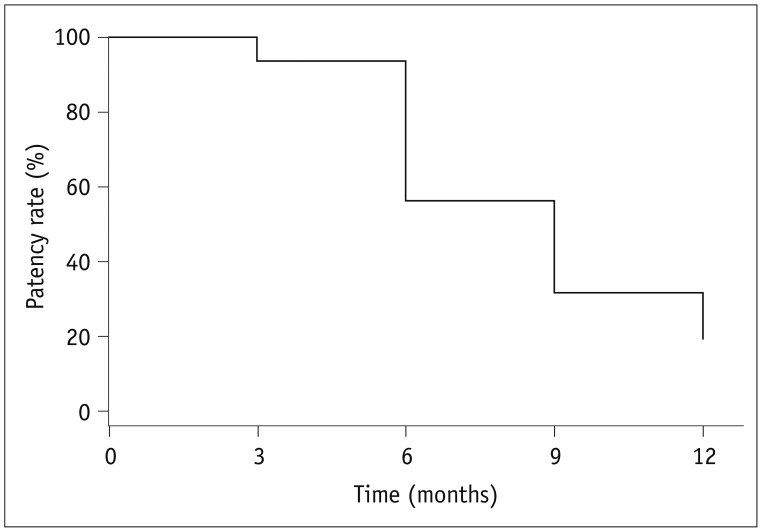
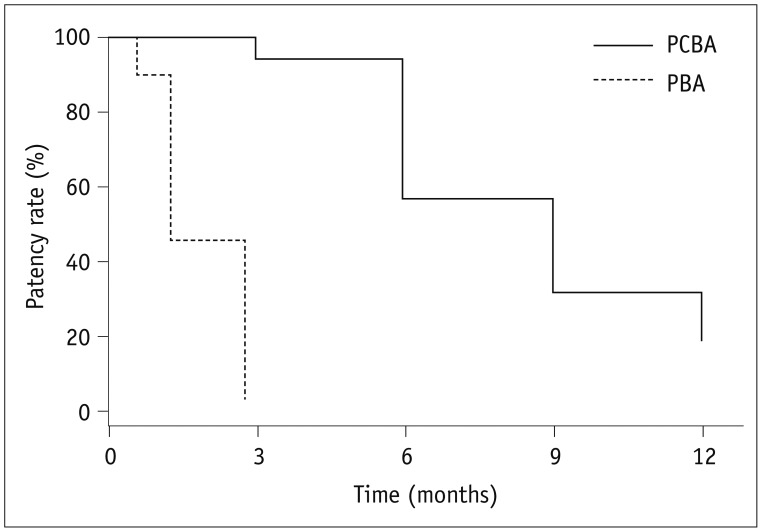
Procedural success was achieved in all 16 cases of central vein stenoses. The mean diameter of the central vein was 3.7 ± 2.4 mm before the procedure vs. 11.4 ± 1.8 mm after the initial procedure. There were no procedure-related complications. The mean diameters of the central veins at 6 months and 12 months were 7.8 ± 1.3 mm and 6.9 ± 2.7 mm, respectively. The primary patency rates at 6 months and 12 months were 93.8% and 31.2%, respectively. One patient had significant restenosis of the central vein at 3 months. The median primary patency period was 9 months for paclitaxel-coated balloons and 2.5 months for the last previous procedure with conventional balloons (p < 0.001).
CONCLUSION
In our limited study, paclitaxel-coated balloons seem to improve the patency rate in cases of early restenosis of central veins. However, a further randomized control trial is necessary.
Keyword
MeSH Terms
Figure
Reference
-
1. Massmann A, Fries P, Obst-Gleditsch K, Minko P, Shayesteh-Kheslat R, Buecker A. Paclitaxel-coated balloon angioplasty for symptomatic central vein restenosis in patients with hemodialysis fistulas. J Endovasc Ther. 2015; 22:74–79. PMID: 25775684.
Article2. Hongsakul K, Rookkapan S, Sungsiri J, Boonsrirat U, Kritpracha B. Pharmacomechanical thrombolysis versus surgical thrombectomy for the treatment of thrombosed haemodialysis grafts. Ann Acad Med Singapore. 2015; 44:66–70. PMID: 25797819.3. Boonsrirat U, Hongsakul K. Pharmacomechanical thrombolysis for the treatment of thrombosed native arteriovenous fistula: a single-center experience. Pol J Radiol. 2014; 79:363–367. PMID: 25343002.
Article4. Bakken AM, Protack CD, Saad WE, Lee DE, Waldman DL, Davies MG. Long-term outcomes of primary angioplasty and primary stenting of central venous stenosis in hemodialysis patients. J Vasc Surg. 2007; 45:776–783. PMID: 17398386.
Article5. Vascular Access 2006 Work Group. Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006; 48(Suppl 1):S176–S247. PMID: 16813989.6. Tepe G, Zeller T, Albrecht T, Heller S, Schwarzwälder U, Beregi JP, et al. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med. 2008; 358:689–699. PMID: 18272892.
Article7. Werk M, Langner S, Reinkensmeier B, Boettcher HF, Tepe G, Dietz U, et al. Inhibition of restenosis in femoropopliteal arteries: paclitaxel-coated versus uncoated balloon: femoral paclitaxel randomized pilot trial. Circulation. 2008; 118:1358–1365. PMID: 18779447.8. Jaff MR, Rosenfield K, Scheinert D, Rocha-Singh K, Benenati J, Nehler M, et al. Drug-coated balloons to improve femoropopliteal artery patency: rationale and design of the LEVANT 2 trial. Am Heart J. 2015; 169:479–485. PMID: 25819854.
Article9. Rosenfield K, Jaff MR, White CJ, Rocha-Singh K, Mena-Hurtado C, Metzger DC, et al. Trial of a paclitaxel-coated balloon for femoropopliteal artery disease. N Engl J Med. 2015; 373:145–153. PMID: 26106946.
Article10. Katsanos K, Karnabatidis D, Kitrou P, Spiliopoulos S, Christeas N, Siablis D. Paclitaxel-coated balloon angioplasty vs. plain balloon dilation for the treatment of failing dialysis access: 6-month interim results from a prospective randomized controlled trial. J Endovasc Ther. 2012; 19:263–272. PMID: 22545894.11. Aruny JE, Lewis CA, Cardella JF, Cole PE, Davis A, Drooz AT, et al. Quality improvement guidelines for percutaneous management of the thrombosed or dysfunctional dialysis access. J Vasc Interv Radiol. 2003; 14(9 Pt 2):S247–S253. PMID: 14514827.
Article12. Kim YC, Won JY, Choi SY, Ko HK, Lee KH, Lee DY, et al. Percutaneous treatment of central venous stenosis in hemodialysis patients: long-term outcomes. Cardiovasc Intervent Radiol. 2009; 32:271–278. PMID: 19194745.
Article13. Surowiec SM, Fegley AJ, Tanski WJ, Sivamurthy N, Illig KA, Lee D, et al. Endovascular management of central venous stenoses in the hemodialysis patient: results of percutaneous therapy. Vasc Endovascular Surg. 2004; 38:349–354. PMID: 15306953.
Article14. Dammers R, de Haan MW, Planken NR, van der Sande FM, Tordoir JH. Central vein obstruction in hemodialysis patients: results of radiological and surgical intervention. Eur J Vasc Endovasc Surg. 2003; 26:317–321. PMID: 14509897.
Article15. Quinn SF, Schuman ES, Demlow TA, Standage BA, Ragsdale JW, Green GS, et al. Percutaneous transluminal angioplasty versus endovascular stent placement in the treatment of venous stenoses in patients undergoing hemodialysis: intermediate results. J Vasc Interv Radiol. 1995; 212:175–180.
Article16. Haage P, Vorwerk D, Piroth W, Schuermann K, Guenther RW. Treatment of hemodialysis-related central venous stenosis or occlusion: results of primary Wallstent placement and follow-up in 50 patients. Radiology. 1999; 212:175–180. PMID: 10405739.
Article17. Anaya-Ayala JE, Smolock CJ, Colvard BD, Naoum JJ, Bismuth J, Lumsden AB, et al. Efficacy of covered stent placement for central venous occlusive disease in hemodialysis patients. J Vasc Surg. 2011; 54:754–759. PMID: 21664095.
Article18. Kundu S, Modabber M, You JM, Tam P, Nagai G, Ting R. Use of PTFE stent grafts for hemodialysis-related central venous occlusions: intermediate-term results. Cardiovasc Intervent Radiol. 2011; 34:949–957. PMID: 21069331.
Article19. Wiskirchen J, Schöber W, Schart N, Kehlbach R, Wersebe A, Tepe G, et al. The effects of paclitaxel on the three phases of restenosis: smooth muscle cell proliferation, migration, and matrix formation: an in vitro study. Invest Radiol. 2004; 39:565–571. PMID: 15308939.20. Axel DI, Kunert W, Göggelmann C, Oberhoff M, Herdeg C, Kuttner A, et al. The effects of paclitaxel on the three phases of restenosis: smooth muscle cell proliferation, migration, and matrix formation: an in vitro study. Circulation. 1997; 96:636–645. PMID: 9244237.21. Portugaller RH, Kalmar PI, Deutschmann H. The eternal tale of dialysis access vessels and restenosis: are drug-eluting balloons the solution? J Vasc Access. 2014; 15:439–447. PMID: 25041917.
Article22. Kitrou PM, Katsanos K, Spiliopoulos S, Karnabatidis D, Siablis D. Drug-eluting versus plain balloon angioplasty for the treatment of failing dialysis access: final results and cost-effectiveness analysis from a prospective randomized controlled trial (NCT01174472). Eur J Radiol. 2015; 84:418–423. PMID: 25575743.
Article23. Verbeeck N, Pillet JC, Toukouki A, Prospert F, Leite S, Mathieu X. Paclitaxel-coated balloon angioplasty of venous stenoses in native dialysis fistulas: primary and secondary patencies at 6 and 12 months. J Belg Soc Radiol. 2016; 100:1–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- No-Reflow Phenomenon During Treatment of Coronary In-Stent Restenosis With a Paclitaxel-Coated Balloon Catheter
- Use of Paclitaxel Coated Drug Eluting Technology to Improve Central Vein Patency for Haemodialysis Access Circuits: Any Benefit?
- Central Vein Stenosis in Hemodialysis Patients
- A Case of Coarctation of the Aorta Treated with Balloon Angioplasty
- Paclitaxel-Coated Balloon versus Plain Balloon Angioplasty for Dysfunctional Autogenous Radiocephalic Arteriovenous Fistulas: A Prospective Randomized Controlled Trial