Korean J Radiol.
2018 Jun;19(3):389-396. 10.3348/kjr.2018.19.3.389.
Diffusion-Weighted Imaging of Upper Abdominal Organs Acquired with Multiple B-Value Combinations: Value of Normalization Using Spleen as the Reference Organ
- Affiliations
-
- 1Department of Radiology, Chonbuk National University Medical School and Hospital, Jeonju 54907, Korea. pichgo@gmail.com
- 2Research Institute of Clinical Medicine of Chonbuk National University, Jeonju 54907, Korea.
- 3Biomedical Research Institute of Chonbuk National University Hospital, Jeonju 54907, Korea.
- 4Department of Surgery, Chonbuk National University Medical School, Jeonju 54907, Korea.
- KMID: 2410809
- DOI: http://doi.org/10.3348/kjr.2018.19.3.389
Abstract
OBJECTIVE
To compare apparent diffusion coefficient (ADC) of the upper abdominal organs acquired with multiple b-value combinations and to investigate usefulness of normalization.
MATERIALS AND METHODS
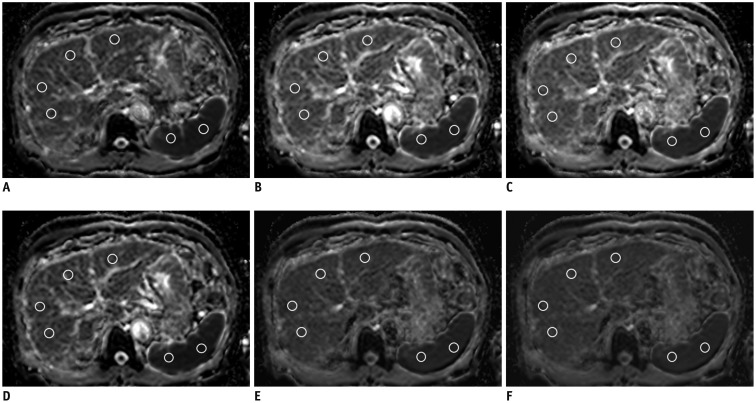
We retrospectively analyzed data, including 3T diffusion-weighted images, of 100 patients (56 men, 44 women; mean age, 63.9) that underwent liver magnetic resonance imaging. An ADC map was derived with the following six b-value combinations: b1 = 0, 50, 400, 800; b2 = 0, 800; b3 = 0, 50, 800; b4 = 0, 400, 800; b5 = 50, 800; and b6 = 50, 400, 800 s/mm2. ADC values of the right liver lobe, left liver lobe, spleen, pancreas, right kidney, and left kidney were measured. ADC values of the spleen were used for normalization. Intraclass correlation coefficients (ICCs), comparison of dependent ICCs, and repeated-measures analysis of variance were used for statistical analysis.
RESULTS
Intraclass correlation coefficients of the original ADC revealed moderate to substantial agreement (0.5145-0.6509), while normalized ADCs revealed almost perfect agreement (0.8014-0.8569). ICC of normalized ADC for all anatomical regions revealed significantly less variability than that of the original ADC (p < 0.05). Coefficient of variance for normalized ADC was significantly lower than that for the original ADC (3.0.3.8% vs. 4.8.8.8%, p < 0.05).
CONCLUSION
Normalization of the ADC values of the upper abdominal organs using the spleen as the reference organ significantly decreased variability in ADC measurement acquired with multiple b-value combinations.
Keyword
MeSH Terms
Figure
Reference
-
1. Soyer P, Kanematsu M, Taouli B, Koh DM, Manfredi R, Vilgrain V, et al. ADC normalization: a promising research track for diffusion-weighted MR imaging of the abdomen. Diagn Interv Imaging. 2013; 94:571–573. PMID: 23731499.
Article2. Koh DM, Takahara T, Imai Y, Collins DJ. Practical aspects of assessing tumors using clinical diffusion-weighted imaging in the body. Magn Reson Med Sci. 2007; 6:211–224. PMID: 18239358.
Article3. Qayyum A. Diffusion-weighted imaging in the abdomen and pelvis: concepts and applications. Radiographics. 2009; 29:1797–1810. PMID: 19959522.
Article4. Thoeny HC, De Keyzer F. Extracranial applications of diffusion-weighted magnetic resonance imaging. Eur Radiol. 2007; 17:1385–1393. PMID: 17206421.
Article5. Afaq A, Andreou A, Koh DM. Diffusion-weighted magnetic resonance imaging for tumour response assessment: why, when and how? Cancer Imaging. 2010; 10(Spec no A):S179–S188. PMID: 20880779.
Article6. Schraml C, Schwenzer NF, Clasen S, Rempp HJ, Martirosian P, Claussen CD, et al. Navigator respiratory-triggered diffusion-weighted imaging in the follow-up after hepatic radiofrequency ablation-initial results. J Magn Reson Imaging. 2009; 29:1308–1316. PMID: 19418557.
Article7. Wybranski C, Zeile M, Löwenthal D, Fischbach F, Pech M, Röhl FW, et al. Value of diffusion weighted MR imaging as an early surrogate parameter for evaluation of tumor response to high-dose-rate brachytherapy of colorectal liver metastases. Radiat Oncol. 2011; 6:43. PMID: 21524305.
Article8. Mannelli L, Kim S, Hajdu CH, Babb JS, Clark TW, Taouli B. Assessment of tumor necrosis of hepatocellular carcinoma after chemoembolization: diffusion-weighted and contrast-enhanced MRI with histopathologic correlation of the explanted liver. AJR Am J Roentgenol. 2009; 193:1044–1052. PMID: 19770328.
Article9. Parikh T, Drew SJ, Lee VS, Wong S, Hecht EM, Babb JS, et al. Focal liver lesion detection and characterization with diffusion-weighted MR imaging: comparison with standard breath-hold T2-weighted imaging. Radiology. 2008; 246:812–822. PMID: 18223123.
Article10. Filipe JP, Curvo-Semedo L, Casalta-Lopes J, Marques MC, Caseiro-Alves F. Diffusion-weighted imaging of the liver: usefulness of ADC values in the differential diagnosis of focal lesions and effect of ROI methods on ADC measurements. MAGMA. 2013; 26:303–312. PMID: 23053714.
Article11. Vallejo Desviat P, Martínez De Vega V, Recio Rodríguez M, Jiménez De La Peña M, Carrascoso Arranz J. [Diffusion MRI in the study of hepatic lesions]. Cir Esp. 2013; 91:9–16. PMID: 22154535.
Article12. Cui Y, Zhang XP, Sun YS, Tang L, Shen L. Apparent diffusion coefficient: potential imaging biomarker for prediction and early detection of response to chemotherapy in hepatic metastases. Radiology. 2008; 248:894–900. PMID: 18710982.
Article13. Koh DM, Scurr E, Collins D, Kanber B, Norman A, Leach MO, et al. Predicting response of colorectal hepatic metastasis: value of pretreatment apparent diffusion coefficients. AJR Am J Roentgenol. 2007; 188:1001–1008. PMID: 17377036.
Article14. Nicholson C, Phillips JM. Ion diffusion modified by tortuosity and volume fraction in the extracellular microenvironment of the rat cerebellum. J Physiol. 1981; 321:225–257. PMID: 7338810.
Article15. Rosenkrantz AB, Oei M, Babb JS, Niver BE, Taouli B. Diffusion-weighted imaging of the abdomen at 3.0 Tesla: image quality and apparent diffusion coefficient reproducibility compared with 1.5 Tesla. J Magn Reson Imaging. 2011; 33:128–135. PMID: 21182130.
Article16. Zhang JL, Sigmund EE, Chandarana H, Rusinek H, Chen Q, Vivier PH, et al. Variability of renal apparent diffusion coefficients: limitations of the monoexponential model for diffusion quantification. Radiology. 2010; 254:783–792. PMID: 20089719.
Article17. Song JS, Kwak HS, Byon JH, Jin GY. Diffusion-weighted MR imaging of upper abdominal organs at different time points: apparent diffusion coefficient normalization using a reference organ. J Magn Reson Imaging. 2017; 45:1494–1501. PMID: 27619627.
Article18. Outwater EK, Siegelman ES, Radecki PD, Piccoli CW, Mitchell DG. Distinction between benign and malignant adrenal masses: value of T1-weighted chemical-shift MR imaging. AJR Am J Roentgenol. 1995; 165:579–583. PMID: 7645474.
Article19. Tsushima Y, Ishizaka H, Matsumoto M. Adrenal masses: differentiation with chemical shift, fast low-angle shot MR imaging. Radiology. 1993; 186:705–709. PMID: 8430178.
Article20. Do RK, Chandarana H, Felker E, Hajdu CH, Babb JS, Kim D, et al. Diagnosis of liver fibrosis and cirrhosis with diffusion-weighted imaging: value of normalized apparent diffusion coefficient using the spleen as reference organ. AJR Am J Roentgenol. 2010; 195:671–676. PMID: 20729445.
Article21. Donner A, Eliasziw M. Sample size requirements for reliability studies. Stat Med. 1987; 6:441–448. PMID: 3629046.
Article22. Donner A, Zou G. Testing the equality of dependent intraclass correlation coefficients. J R Stat Soc Ser D (The Statistician). 2002; 51:367–379.
Article23. Kim SY, Lee SS, Park B, Kim N, Kim JK, Park SH, et al. Reproducibility of measurement of apparent diffusion coefficients of malignant hepatic tumors: effect of DWI techniques and calculation methods. J Magn Reson Imaging. 2012; 36:1131–1138. PMID: 22777895.
Article24. Braithwaite AC, Dale BM, Boll DT, Merkle EM. Short- and midterm reproducibility of apparent diffusion coefficient measurements at 3.0-T diffusion-weighted imaging of the abdomen. Radiology. 2009; 250:459–465. PMID: 19095786.
Article25. Corona-Villalobos CP, Pan L, Halappa VG, Bonekamp S, Lorenz CH, Eng J, et al. Agreement and reproducibility of apparent diffusion coefficient measurements of dual-b-value and multi-b-value diffusion-weighted magnetic resonance imaging at 1.5 Tesla in phantom and in soft tissues of the abdomen. J Comput Assist Tomogr. 2013; 37:46–51. PMID: 23321832.
Article26. Miquel ME, Scott AD, Macdougall ND, Boubertakh R, Bharwani N, Rockall AG. In vitro and in vivo repeatability of abdominal diffusion-weighted MRI. Br J Radiol. 2012; 85:1507–1512. PMID: 22674704.27. Padhani AR, Liu G, Koh DM, Chenevert TL, Thoeny HC, Takahara T, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009; 11:102–125. PMID: 19186405.
Article28. Song JS, Hwang SB, Chung GH, Jin GY. Intra-individual, inter-vendor comparison of diffusion-weighted MR imaging of upper abdominal organs at 3.0 Tesla with an emphasis on the value of normalization with the spleen. Korean J Radiol. 2016; 17:209–217. PMID: 26957905.
Article29. Kim T, Murakami T, Takahashi S, Hori M, Tsuda K, Nakamura H. Diffusion-weighted single-shot echoplanar MR imaging for liver disease. AJR Am J Roentgenol. 1999; 173:393–398. PMID: 10430143.
Article30. Papanikolaou N, Gourtsoyianni S, Yarmenitis S, Maris T, Gourtsoyiannis N. Comparison between two-point and four-point methods for quantification of apparent diffusion coefficient of normal liver parenchyma and focal lesions. value of normalization with spleen. Eur J Radiol. 2010; 73:305–309. PMID: 19091503.
Article31. Taouli B, Thakur RK, Mannelli L, Babb JS, Kim S, Hecht EM, et al. Renal lesions: characterization with diffusion-weighted imaging versus contrast-enhanced MR imaging. Radiology. 2009; 251:398–407. PMID: 19276322.
Article32. Park MY, Byun JY. Understanding the mathematics involved in calculating apparent diffusion coefficient maps. AJR Am J Roentgenol. 2012; 199:W784. PMID: 23169755.
Article33. Donati OF, Chong D, Nanz D, Boss A, Froehlich JM, Andres E, et al. Diffusion-weighted MR imaging of upper abdominal organs: field strength and intervendor variability of apparent diffusion coefficients. Radiology. 2014; 270:454–463. PMID: 24471390.
Article34. Chen X, Qin L, Pan D, Huang Y, Yan L, Wang G, et al. Liver diffusion-weighted MR imaging: reproducibility comparison of ADC measurements obtained with multiple breath-hold, free-breathing, respiratory-triggered, and navigator-triggered techniques. Radiology. 2014; 271:113–125. PMID: 24475860.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intra-Individual, Inter-Vendor Comparison of Diffusion-Weighted MR Imaging of Upper Abdominal Organs at 3.0 Tesla with an Emphasis on the Value of Normalization with the Spleen
- Diffusion-Weighted Magnetic Resonance Imaging of Spine
- A Case of Wandering Spleen in a Patient Who Presented at the ED with Mild Abdominal Pain in the Left Upper Quadrant
- Torsion of a Wandering Spleen Treated by Laparoscopic Surgery: A Case Report
- Diffusion-Weighted MR Imaging of Upper Abdomen: Comparison of Breath-Hold, Free-Breathing, and Respiratory-Triggered Techniques