Brain Tumor Res Treat.
2018 Apr;6(1):22-30. 10.14791/btrt.2018.6.e1.
Prognostic Evaluation of Neurological Assessment of the Neuro-Oncology Scale in Glioblastoma Patients
- Affiliations
-
- 1Division of Neuro-Oncology and Department of Neurosurgery, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea. yzkim@skku.edu
- KMID: 2410234
- DOI: http://doi.org/10.14791/btrt.2018.6.e1
Abstract
- BACKGROUND
The aims of this study were to investigate the role of the Neurological Assessment of Neuro-Oncology (NANO) scale in predicting the prognosis of patients with glioblastoma, and compare these results to predicted data of the Karnofsky Performance Scale (KPS), and Eastern Cooperative Oncology Group (ECOG)/World Health Organization (WHO) performance status. Additionally, we examined other prognostic factors in glioblastoma patients.
METHODS
The medical records of 76 patients with a new diagnosis of histologically ascertained glioblastoma in the period from January 2002 to December 2015 at the authors' institution were retrospectively reviewed. Clinical factors, including epidemiologic, radiologic, and therapeutic values were reviewed as well as the performance status assessed by the KPS, ECOG/WHO performance status, and NANO scale.
RESULTS
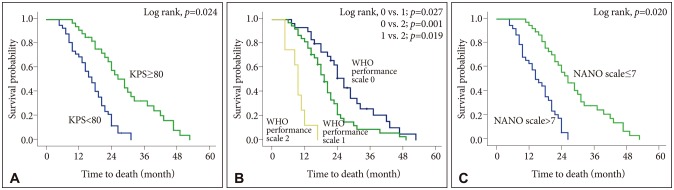
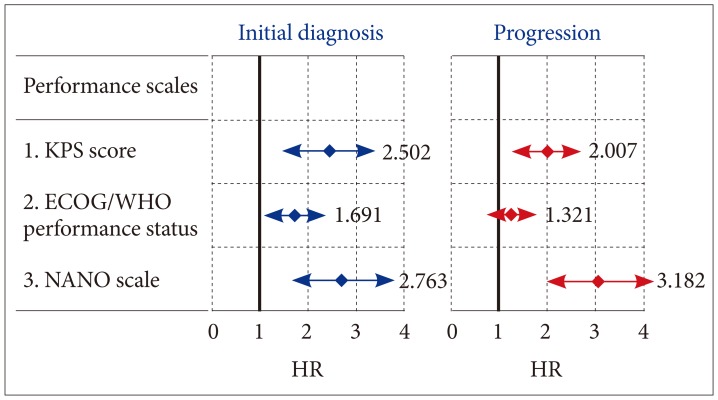
The mean overall survival was 19.8 months (95% confidence interval 15.2-25.4 months). At initial diagnosis, the mean value [±standard deviation (SD)] of KPS score, ECOG/WHO performance status, and NANO scale were 81 (±7.4), 1.3 (±0.6), and 7.3 (±3.8), respectively. Multivariate analysis for predicting survival showed odds ratios of KPS score, ECOG/WHO performance status, and NANO scale were 2.502 (≥80 vs. < 80; p=0.024), 1.691 (0-1 vs. 2-5; p=0.047), and 2.763 (0-7 vs. 8-23; p=0.020), respectively. At the time of progression, the mean value (±SD) of KPS score, ECOG/WHO performance status, and NANO scale were 69 (±8.2), 1.6 (±0.7), and 11.4 (±4.2), respectively; multivariate analysis for predicting survival showed that the odd ratios for KPS score, ECOG/WHO performance status, and NANO scale were 2.007 (≥80 vs. < 80; p=0.035), 1.321 (0-1 vs. 2-5; p=0.143), and 3.182 (0-7 vs. 8-23; p=0.002), respectively.
CONCLUSION
The NANO scale provided a more detailed and objective measure of neurologic function than that currently used for predicting the prognosis of glioblastoma patients, especially at the time of progression.
Keyword
MeSH Terms
Figure
Reference
-
1. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014; 16(Suppl 4):iv1–iv63. PMID: 25304271.
Article2. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Ellison DW, Figarella-Branger D. Glioblastoma, IDH-wild-types. In : Louise DN, Brate DJ, Ohgaki H, editors. WHO Classification of Tumours of the Central Nervous System. Rev 4th ed. Lyon: IARC Press;2016. p. 28–45.3. Dho YS, Jung KW, Ha J, et al. An updated nationwide epidemiology of primary brain tumors in Republic of Korea, 2013. Brain Tumor Res Treat. 2017; 5:16–23. PMID: 28516074.
Article4. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005; 352:997–1003. PMID: 15758010.5. Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009; 10:459–466. PMID: 19269895.6. Marina O, Suh JH, Reddy CA, et al. Treatment outcomes for patients with glioblastoma multiforme and a low Karnofsky Performance Scale score on presentation to a tertiary care institution. J Neurosurg. 2011; 115:220–229. PMID: 21548745.
Article7. Lamborn KR, Chang SM, Prados MD. Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neuro Oncol. 2004; 6:227–235. PMID: 15279715.8. Li J, Wang M, Won M, et al. Validation and simplification of the Radiation Therapy Oncology Group recursive partitioning analysis classification for glioblastoma. Int J Radiat Oncol Biol Phys. 2011; 81:623–630. PMID: 20888136.
Article9. Gorlia T, van den Bent MJ, Hegi ME, et al. Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981-22981/CE.3. Lancet Oncol. 2008; 9:29–38. PMID: 18082451.
Article10. McGirt MJ, Chaichana KL, Gathinji M, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009; 110:156–162. PMID: 18847342.
Article11. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982; 5:649–655. PMID: 7165009.
Article12. Young J, Badgery-Parker T, Dobbins T, et al. Comparison of ECOG/WHO performance status and ASA score as a measure of functional status. J Pain Symptom Manage. 2015; 49:258–264. PMID: 24996034.
Article13. Nayak L, DeAngelis LM, Brandes AA, et al. The Neurologic Assessment in Neuro-Oncology (NANO) scale: a tool to assess neurologic function for integration into the Response Assessment in Neuro-Oncology (RANO) criteria. Neuro Oncol. 2017; 19:625–635. PMID: 28453751.
Article14. Karayan-Tapon L, Quillien V, Guilhot J, et al. Prognostic value of O6-methylguanine-DNA methyltransferase status in glioblastoma patients, assessed by five different methods. J Neurooncol. 2010; 97:311–322. PMID: 19841865.
Article15. Palmisano WA, Divine KK, Saccomanno G, et al. Predicting lung cancer by detecting aberrant promoter methylation in sputum. Cancer Res. 2000; 60:5954–5958. PMID: 11085511.16. Kim J, Lee SH, Jang JH, Kim MS, Lee EH, Kim YZ. Increased expression of the histone H3 lysine 4 methyltransferase MLL4 and the histone H3 lysine 27 demethylase UTX prolonging the overall survival of patients with glioblastoma and a methylated MGMT promoter. J Neurosurg. 2017; 126:1461–1471. PMID: 27367247.
Article17. Provenzale JM, Ison C, Delong D. Bidimensional measurements in brain tumors: assessment of interobserver variability. AJR Am J Roentgenol. 2009; 193:W515–W522. PMID: 19933626.
Article18. Eng J. Receiver operating characteristic analysis: a primer. Acad Radiol. 2005; 12:909–916. PMID: 16039544.19. Dhermain FG, Hau P, Lanfermann H, Jacobs AH, van den Bent MJ. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol. 2010; 9:906–920. PMID: 20705518.
Article20. Jaspan T, Morgan PS, Warmuth-Metz M, et al. Response assessment in pediatric neuro-oncology: implementation and expansion of the RANO criteria in a randomized phase II trial of pediatric patients with newly diagnosed high-grade gliomas. AJNR Am J Neuroradiol. 2016; 37:1581–1587. PMID: 27127006.
Article21. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neurooncology working group. J Clin Oncol. 2010; 28:1963–1972. PMID: 20231676.
Article22. Chang SM, Wen PY, Vogelbaum MA, Macdonald DR, van den Bent MJ. Response Assessment in Neuro-Oncology (RANO): more than imaging criteria for malignant glioma. Neuro-Oncol Prac. 2015; 2:205–209.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Supramaximal Resection for Glioblastoma: Redefining the Extent of Resection Criteria and Its Impact on Survival
- Yesterdays, Todays, and Tomorrows—Korean Society for Pediatric Neuro-Oncology*
- Altered Histone Modifications in Gliomas
- Radiomics and Deep Learning from Research to Clinical Workflow: Neuro-Oncologic Imaging
- Postoperative Radiation Therapy of Astrocytoma and Glioblastoma Multiforme