Korean J Physiol Pharmacol.
2018 May;22(3):349-360. 10.4196/kjpp.2018.22.3.349.
Anti-apoptotic effects of autophagy via ROS regulation in microtubule-targeted and PDGF-stimulated vascular smooth muscle cells
- Affiliations
-
- 1Department of Pharmacology, Chungnam National University College of Pharmacy, Daejeon 34134, Korea. cm8r@cnu.ac.kr
- 2Institute of Drug Research & Development, Chungnam National University, Daejeon 34134, Korea.
- KMID: 2410099
- DOI: http://doi.org/10.4196/kjpp.2018.22.3.349
Abstract
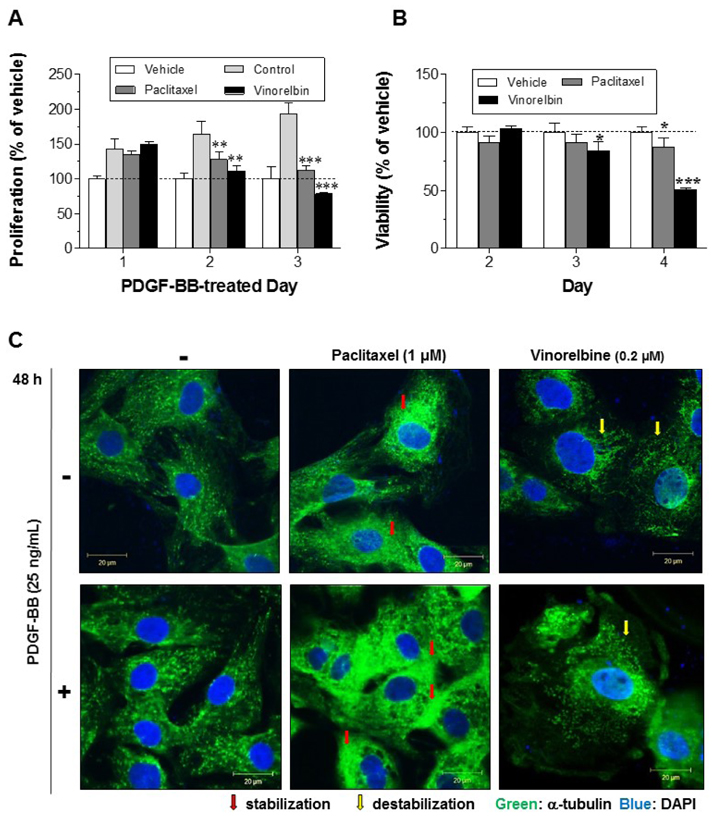
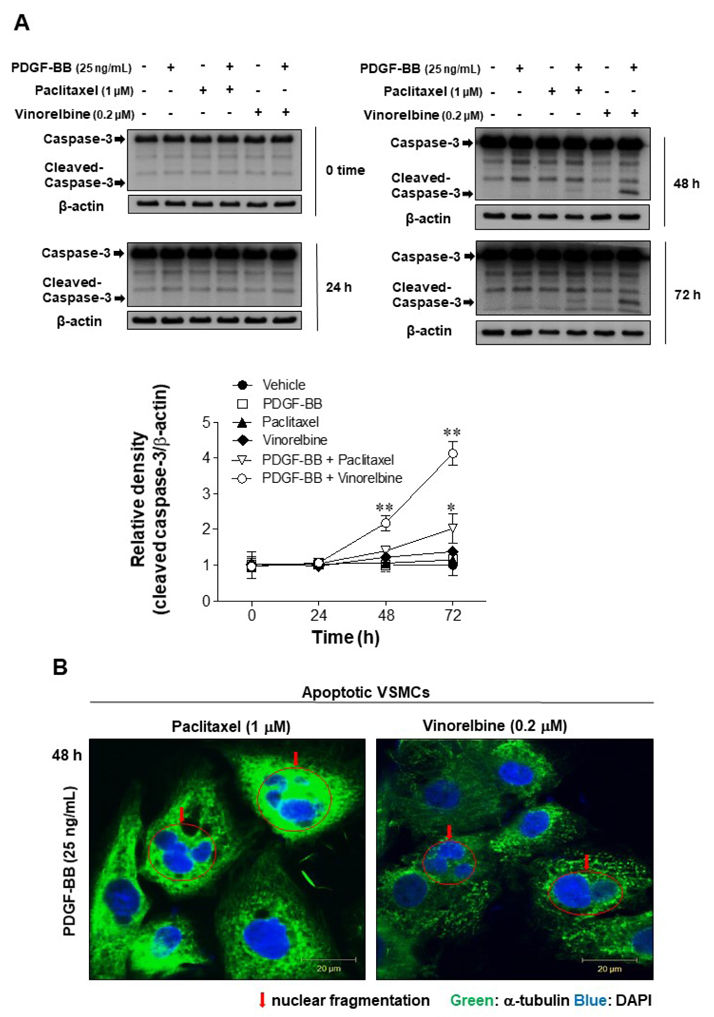
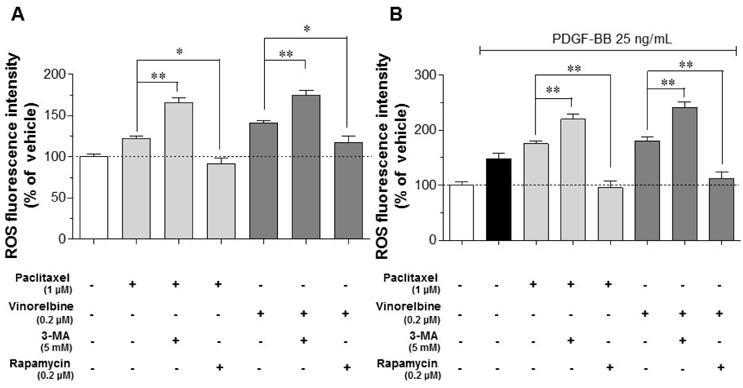
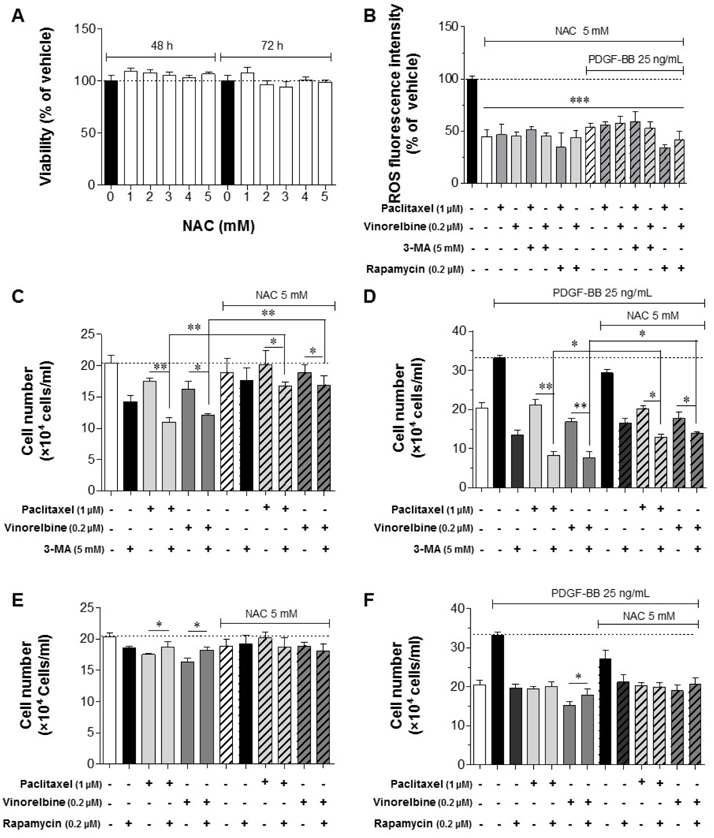
- Autophagy has been studied as a therapeutic strategy for cardiovascular diseases. However, insufficient studies have been reported concerning the influence of vascular smooth muscle cells (VSMCs) through autophagy regulation. The aim of the present study was to determine the effects of VSMCs on the regulation of autophagy under in vitro conditions similar to vascular status of the equipped microtubule target agent-eluting stent and increased release of platelet-derived growth factor-BB (PDGF-BB). Cell viability and proliferation were measured using MTT and cell counting assays. Immunofluorescence using an anti-α-tubulin antibody was performed to determine microtubule dynamic formation. Cell apoptosis was measured by cleavage of caspase-3 using western blot analysis, and by nuclear fragmentation using a fluorescence assay. Autophagy activity was assessed by microtubule-associated protein light chain 3-II (LC-II) using western blot analysis. Levels of intracellular reactive oxygen species (ROS) were measured using H₂DCFDA. The proliferation and viability of VSMCs were inhibited by microtubule regulation. Additionally, microtubule-regulated and PDGF-BB-stimulated VSMCs increased the cleavage of caspase-3 more than only the microtubule-regulated condition, similar to that of LC3-II, implying autophagy. Inhibitory autophagy of microtubule-regulated and PDGF-BB-stimulated VSMCs resulted in low viability. However, enhancement of autophagy maintained survival through the reduction of ROS. These results suggest that the apoptosis of conditioned VSMCs is decreased by the blocking generation of ROS via the promotion of autophagy, and proliferation is also inhibited. Thus, promoting autophagy as a therapeutic target for vascular restenosis and atherosclerosis may be a good strategy.
MeSH Terms
Figure
Cited by 1 articles
-
Inhibitory Effect of Ginsenosides Rh1 and Rg2 on Oxidative Stress in LPS-Stimulated RAW 264.7 Cells
Yujin Jin, Naehwan Baek, Soyoung Back, Chang-Seon Myung, Kyung-Sun Heo
J Bacteriol Virol. 2018;48(4):156-165. doi: 10.4167/jbv.2018.48.4.156.
Reference
-
1. Chistiakov DA, Orekhov AN, Bobryshev YV. Vascular smooth muscle cell in atherosclerosis. Acta Physiol (Oxf). 2015; 214:33–50.
Article2. Shioi A, Ikari Y. Plaque calcification during atherosclerosis progression and regression. J Atheroscler Thromb. 2018; 25:294–303.
Article3. van der Wal AC, Becker AE. Atherosclerotic plaque rupture— pathologic basis of plaque stability and instability. Cardiovasc Res. 1999; 41:334–344.4. Ramji DP, Davies TS. Cytokines in atherosclerosis: key players in all stages of disease and promising therapeutic targets. Cytokine Growth Factor Rev. 2015; 26:673–685.
Article5. Ross R. Growth factors in the pathogenesis of atherosclerosis. Acta Med Scand Suppl. 1987; 715:33–38.
Article6. Raines EW, Ross R. Multiple growth factors are associated with lesions of atherosclerosis: specificity or redundancy? Bioessays. 1996; 18:271–282.
Article7. Cagnin S, Biscuola M, Patuzzo C, Trabetti E, Pasquali A, Laveder P, Faggian G, Iafrancesco M, Mazzucco A, Pignatti PF, Lanfranchi G. Reconstruction and functional analysis of altered molecular pathways in human atherosclerotic arteries. BMC Genomics. 2009; 10:13.
Article8. Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008; 22:1276–1312.
Article9. Gershlick A, De Scheerder I, Chevalier B, Stephens-Lloyd A, Camenzind E, Vrints C, Reifart N, Missault L, Goy JJ, Brinker JA, Raizner AE, Urban P, Heldman AW. Inhibition of restenosis with a paclitaxel-eluting, polymer-free coronary stent: the European evaLUation of pacliTaxel Eluting Stent (ELUTES) trial. Circulation. 2004; 109:487–493.10. Alfonso F, Byrne RA, Rivero F, Kastrati A. Current treatment of instent restenosis. J Am Coll Cardiol. 2014; 63:2659–2673.
Article11. Sifringer M, Bendix I, Börner C, Endesfelder S, von Haefen C, Kalb A, Holifanjaniaina S, Prager S, Schlager GW, Keller M, Jacotot E, Felderhoff-Mueser U. Prevention of neonatal oxygen-induced brain damage by reduction of intrinsic apoptosis. Cell Death Dis. 2012; 3:e250.
Article12. Kobayashi S. Choose delicately and reuse adequately: the newly revealed process of autophagy. Biol Pharm Bull. 2015; 38:1098–1103.
Article13. Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011; 469:323–335.
Article14. Mizushima N, Yoshimori T, Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol. 2011; 27:107–132.
Article15. Burman C, Ktistakis NT. Autophagosome formation in mammalian cells. Semin Immunopathol. 2010; 32:397–413.
Article16. Nussenzweig SC, Verma S, Finkel T. The role of autophagy in vascular biology. Circ Res. 2015; 116:480–488.
Article17. De Meyer GR, Grootaert MO, Michiels CF, Kurdi A, Schrijvers DM, Martinet W. Autophagy in vascular disease. Circ Res. 2015; 116:468–479.
Article18. Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008; 132:27–42.
Article19. Salabei JK, Hill BG. Autophagic regulation of smooth muscle cell biology. Redox Biol. 2015; 4:97–103.
Article20. Grootaert MO, da Costa Martins PA, Bitsch N, Pintelon I, De Meyer GR, Martinet W, Schrijvers DM. Defective autophagy in vascular smooth muscle cells accelerates senescence and promotes neointima formation and atherogenesis. Autophagy. 2015; 11:2014–2032.
Article21. Cho YS, Yen CN, Shim JS, Kang DH, Kang SW, Liu JO, Kwon HJ. Antidepressant indatraline induces autophagy and inhibits restenosis via suppression of mTOR/S6 kinase signaling pathway. Sci Rep. 2016; 6:34655.
Article22. Park HS, Quan KT, Han JH, Jung SH, Lee DH, Jo E, Lim TW, Heo KS, Na M, Myung CS. Rubiarbonone C inhibits platelet-derived growth factor-induced proliferation and migration of vascular smooth muscle cells through the focal adhesion kinase, MAPK and STAT3 Tyr705 signalling pathways. Br J Pharmacol. 2017; 174:4140–4154.23. Lee JJ, Zhang WY, Yi H, Kim Y, Kim IS, Shen GN, Song GY, Myung CS. Anti-proliferative actions of 2-decylamino-5,8-dimethoxy-1,4-naphthoquinone in vascular smooth muscle cells. Biochem Biophys Res Commun. 2011; 411:213–218.
Article24. Choi KW, Park HJ, Jung DH, Kim TW, Park YM, Kim BO, Sohn EH, Moon EY, Um SH, Rhee DK, Pyo S. Inhibition of TNF-α-induced adhesion molecule expression by diosgenin in mouse vascular smooth muscle cells via downregulation of the MAPK, Akt and NF-κB signaling pathways. Vascul Pharmacol. 2010; 53:273–280.
Article25. Horwitz SB. Taxol (paclitaxel): mechanisms of action. Ann Oncol. 1994; 5:Suppl 6. S3–S6.26. Fukuoka K, Arioka H, Iwamoto Y, Fukumoto H, Kurokawa H, Ishida T, Tomonari A, Suzuki T, Usuda J, Kanzawa F, Saijo N, Nishio K. Mechanism of the radiosensitization induced by vinorelbine in human non-small cell lung cancer cells. Lung Cancer. 2001; 34:451–460.
Article27. Perez EA. Microtubule inhibitors: differentiating tubulin-inhibiting agents based on mechanisms of action, clinical activity, and resistance. Mol Cancer Ther. 2009; 8:2086–2095.
Article28. Wang RC, Chen X, Parissenti AM, Joy AA, Tuszynski J, Brindley DN, Wang Z. Sensitivity of docetaxel-resistant MCF-7 breast cancer cells to microtubule-destabilizing agents including vinca alkaloids and colchicine-site binding agents. PLoS One. 2017; 12:e0182400.
Article29. Perez-Vizcaino F, Bishop-Bailley D, Lodi F, Duarte J, Cogolludo A, Moreno L, Bosca L, Mitchell JA, Warner TD. The flavonoid quercetin induces apoptosis and inhibits JNK activation in intimal vascular smooth muscle cells. Biochem Biophys Res Commun. 2006; 346:919–925.
Article30. Tixeira R, Caruso S, Paone S, Baxter AA, Atkin-Smith GK, Hulett MD, Poon IK. Defining the morphologic features and products of cell disassembly during apoptosis. Apoptosis. 2017; 22:475–477.
Article31. Petiot A, Ogier-Denis E, Blommaart EF, Meijer AJ, Codogno P. Distinct classes of phosphatidylinositol 3’-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J Biol Chem. 2000; 275:992–998.
Article32. Kim YC, Guan KL. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest. 2015; 125:25–32.
Article33. García-Arencibia M, Hochfeld WE, Toh PP, Rubinsztein DC. Autophagy, a guardian against neurodegeneration. Semin Cell Dev Biol. 2010; 21:691–698.
Article34. Li BH, Yin YW, Liu Y, Pi Y, Guo L, Cao XJ, Gao CY, Zhang LL, Li JC. TRPV1 activation impedes foam cell formation by inducing autophagy in oxLDL-treated vascular smooth muscle cells. Cell Death Dis. 2014; 5:e1182.
Article35. Hill BG, Haberzettl P, Ahmed Y, Srivastava S, Bhatnagar A. Unsaturated lipid peroxidation-derived aldehydes activate autophagy in vascular smooth-muscle cells. Biochem J. 2008; 410:525–534.
Article36. Salabei JK, Cummins TD, Singh M, Jones SP, Bhatnagar A, Hill BG. PDGF-mediated autophagy regulates vascular smooth muscle cell phenotype and resistance to oxidative stress. Biochem J. 2013; 451:375–388.
Article37. Cj P, Hv E, Vijayakurup V, R Menon G, Nair S, Gopala S. High LC3/Beclin expression correlates with poor survival in glioma: a definitive role for autophagy as evidenced by in vitro autophagic flux. Pathol Oncol Res. 2017; DOI: 10.1007/s12253-017-0310-7. [Epub ahead of print].
Article38. van Meerloo J, Kaspers GJ, Cloos J. Cell sensitivity assays: the MTT assay. Methods Mol Biol. 2011; 731:237–245.
Article39. Zhang Y, Xia G, Zhang Y, Liu J, Liu X, Li W, Lv Y, Wei S, Liu J, Quan J. Palmitate induces VSMC apoptosis via toll like receptor (TLR)4/ ROS/p53 pathway. Atherosclerosis. 2017; 263:74–81.40. Cao YN, Zheng LL, Wang D, Liang XX, Gao F, Zhou XL. Recent advances in microtubule-stabilizing agents. Eur J Med Chem. 2018; 143:806–828.
Article41. Hugle M, Belz K, Fulda S. Identification of synthetic lethality of PLK1 inhibition and microtubule-destabilizing drugs. Cell Death Differ. 2015; 22:1946–1956.
Article42. Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999; 6:99–104.
Article43. Sollott SJ, Cheng L, Pauly RR, Jenkins GM, Monticone RE, Kuzuya M, Froehlich JP, Crow MT, Lakatta EG, Rowinsky EK, et al. Taxol inhibits neointimal smooth muscle cell accumulation after angioplasty in the rat. J Clin Invest. 1995; 95:1869–1876.
Article44. Mariño G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol. 2014; 15:81–94.
Article45. Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N. Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta. 2013; 1833:3448–3459.
Article46. Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011; 18:571–580.
Article47. Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010; 48:749–762.
Article48. Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000; 5:415–418.49. Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995; 270:296–299.50. Kappert K, Sparwel J, Sandin A, Seiler A, Siebolts U, Leppänen O, Rosenkranz S, Ostman A. Antioxidants relieve phosphatase inhibition and reduce PDGF signaling in cultured VSMCs and in restenosis. Arterioscler Thromb Vasc Biol. 2006; 26:2644–2651.
Article51. Poillet-Perez L, Despouy G, Delage-Mourroux R, Boyer-Guittaut M. Interplay between ROS and autophagy in cancer cells, from tumor initiation to cancer therapy. Redox Biol. 2015; 4:184–192.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Artemisinin attenuates platelet-derived growth factor BB-induced migration of vascular smooth muscle cells
- Inhibition of rac1 Reduces PDGF-induced Reactive Oxygen Species and Proliferation in Vascular Smooth Muscle Cells
- The Role of Autophagy in the Pathogenesis of Atherosclerosis
- Mechanisms Involved in the Inhibitory Effects of Mycophenolic Acid on the PDGF-induced Proliferation of Vascular Smooth Muscle Cells
- Influence of Endothelin-1 on Cultured Vascular Smooth Muscle Cell Proliferation