Nat Prod Sci.
2018 Mar;24(1):66-70. 10.20307/nps.2018.24.1.66.
Chemical Composition and Antimicrobial Efficacy of Helminthostachys zeylanica against Foodborne Bacillus cereus
- Affiliations
-
- 1Universiti Kuala Lumpur, Malaysian Institute of Chemical and Bioengineering Technology, Lot 1988 Kawasan Perindustrian Bandar Vendor, Taboh Naning, 78000 Alor Gajah, Melaka, Malaysia. wytong@unikl.edu.my
- 2School of Distance Education, Universiti Sains Malaysia, 11800 Minden, Penang, Malaysia.
- KMID: 2409612
- DOI: http://doi.org/10.20307/nps.2018.24.1.66
Abstract
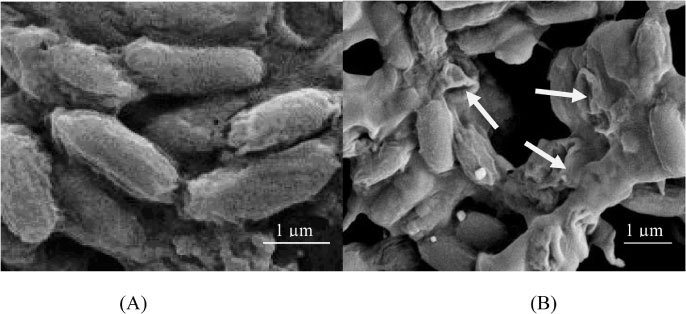
- Helminthostachys zeylanica is a rare plant grows in lightly shaded areas. The fern was traditionally used as antipyretic and antiphlogistic agents. This study was aimed to evaluate the antibacterial potential of H. zeylanica on foodborne Bacillus cereus. The chemical composition of its ethanolic extract was also determined. The plant samples were collected at Kampung Kebun Relong, Kedah, Malaysia. The ethanolic extract showed significant inhibitory activity on B. cereus with a sizeable clear zone detected on disc diffusion assay. On broth microdilution assay, the MIC of the extract on B. cereus was 6.25 mg/ml and the MBC was 12.5 mg/ml. The inhibitory activity of the extract on B. cereus was bactericidal. In the growth dynamic study, the antibacterial efficacy of the extract was concentration dependent, where a lower colony forming unit count was obtained with increased extract concentration. The SEM micrograph of extract treated B. cereus cells showed invaginations of cell wall. The bacterial cell structure collapsed after 24 h exposure to the extract. The GCMS analysis of the extract showed that the major constituents of the extract were phenol (36.26%) and quercetin (29.70%). This study is important as it shows the potential use of H. zeylanica as an effective agent to control B. cereus related infections.
MeSH Terms
Figure
Reference
-
1. Batubara I, Mitsunaga T, Ohashi HJ. Wood Sci. 2009; 55:230–235.2. Huang YC, Hwang TL, Chang CS, Yang YL, Shen CN, Liao WY, Liaw CC. J Nat Prod. 2009; 72:1273–1278.3. Suja SR, Latha PG, Pushpangadan P, Rajasekharan SJ. Ethnopharmacol. 2004; 92:61–66.4. Malorny B, Tassios PT, Rådström P, Cook N, Wagner M, Hoorfar J. Int J Food Microbiol. 2003; 83:39–48.5. Bennett SD, Walsh KA, Gould LH. Clin Infect Dis. 2013; 57:425–433.6. Lücking G, Frenzel E, Rütschle A, Marxen S, Stark TD, Hofmann T, Ehling-Schulz M. Front Microbiol. 2015; 6:113–116.7. Abdul-Mutalib NA, Syafinaz AN, Sakai K, Shirai Y. Int Food Res J. 2015; 22:896–901.8. Sandra A, Afsah-Hejri L, Tunung R, Tuan Zainazor TC, Tang JYH, Ghazali FM, Nakaguchi Y, Nishibuchi M, Son R. Int Food Res J. 2012; 19:829–836.9. Abdul-Mutalib NA, Amin Nordin S, Osman M, Ishida N, Tashiro K, Sakai K, Tashiro Y, Maeda T, Shirai Y. Int J Food Microbiol. 2015; 200:57–65.10. Tong WY, Zaadah JN, Tan WN, Melati K, Latiffah Z, Darah I. Annu Res Rev Biol. 2014; 4:1490–1501.11. Azmir J, Zaidul ISM, Rahman MM, Sharif KM, Mohamed A, Sahena F, Jahurul MHA, Ghafoor K, Norulaini NAN, Omar AKM. J Food Eng. 2013; 11:426–436.12. Nath K, Bhattacharya MK, Sen A, Kar S. Int J Pharmacogn Phytochem. 2013; 28:1169–1172.13. Puri M, Sharma D, Barrow CJ. Trends Biotechnol. 2012; 30:37–44.14. Wong JY, Matanjun P, Ooi YBH, Chia KF. Int J Food Prop. 2014; 17:1763–1778.15. Cushnie TT, Lamb AJ. Int J Antimicrob Agent. 2005; 26:343–356.16. Banfi E, Scialino G, Monti-Bragadin CJ. Antimicrob Chemotherap. 2003; 52:796–800.17. French GL. J Antimicrob Chemother. 2006; 58:1107–1117.18. Klepser ME, Ernst EJ, Petzold CR, Rhomberg P, Doern GV. Antimicrob Agents Chemother. 2001; 45:673–678.19. Liasi SA, Azmi TI, Hassan MD, Shuhaimi M, Rosfarizan M, Ariff AB. Malays J Microbiol. 2009; 5:33–37.20. Bendini A, Cerretani L, Pizzolante L, Toschi TG, Guzzo F, Ceoldo S, Marconi AM, Andreetta F, Levi M. Eur Food Res Tech. 2006; 223:102–109.21. Ultee A, Bennik MHJ, Moezelaar R. Appl Environ Microbiol. 2002; 68:1561–1568.22. Gatto MT, Falcocchio S, Grippa E, Mazzanti G, Battinelli L, Nicolosi G, Saso L. Bioorg Med Chem. 2002; 10:269–272.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of Kudoa septempunctata as a cause of foodborne illness and its associated differential diagnosis
- Effect of Agrimonia Pilosa Ledeb. Extract on the Growth of Food-Borne Pathogens
- Antimicrobial Activities of Omija Extracts Against Bacillus cereus and Escherchia coli
- Whole genome sequence analyses of thermotolerant Bacillus sp. isolates from food
- A Case of Bacillus Cereus Infection with Pneumonia and Bactermia