J Korean Med Assoc.
2018 Feb;61(2):116-122. 10.5124/jkma.2018.61.2.116.
Comprehensive review and update on herpes zoster
- Affiliations
-
- 1Department of Dermatology, Seoul St. Mary's Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea. yymmpark6301@hotmail.com
- KMID: 2409019
- DOI: http://doi.org/10.5124/jkma.2018.61.2.116
Abstract
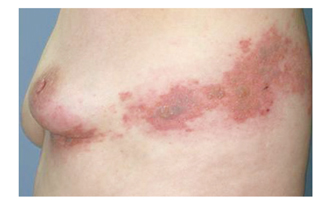
- Herpes zoster (HZ) is the result of reactivation and multiplication of latent varicella zoster virus that persisted in latent form within the sensory ganglia following an earlier attack of varicella. It occurs most frequently in older adults and immunosuppressed individuals. Classically, the rash presents as painful, erythematous, maculopapular, and vesicular lesions that typically involve single dermatome, and usually do not cross the midline. The diagnosis is mainly made clinically, except in patients with atypical manifestations in which laboratory virologic testing is required. HZ has been associated with several complications, of which postherpetic neuralgia is the most common and debilitating. The treatment of HZ includes the use of antiviral agents, analgesics for control of acute zoster pain, good skin care for healing, and prevention of secondary bacterial infection. Antiviral agents should be started within 72 hours of onset to reduce the severity of the infection, the duration of the eruptive phase, and the intensity of acute pain. The options for treating postherpetic neuralgia include lidocaine patch, high dose capsaicin patch, gabapentin, pregabalin, opioids, and tricyclic antidepressants. A live attenuated zoster vaccine reduces the incidence of by one-half and the incidence of postherpetic neuralgia by two-thirds. We herein review the recent data on the epidemiology, pathophysiology, diagnosis and management of HZ including HZ vaccine.
MeSH Terms
-
Acute Pain
Adult
Analgesics
Analgesics, Opioid
Antidepressive Agents, Tricyclic
Antiviral Agents
Bacterial Infections
Capsaicin
Chickenpox
Diagnosis
Epidemiology
Exanthema
Ganglia, Sensory
Herpes Zoster Vaccine
Herpes Zoster*
Herpesvirus 3, Human
Humans
Incidence
Lidocaine
Neuralgia, Postherpetic
Pregabalin
Skin Care
Analgesics
Analgesics, Opioid
Antidepressive Agents, Tricyclic
Antiviral Agents
Capsaicin
Herpes Zoster Vaccine
Lidocaine
Pregabalin
Figure
Reference
-
1. Oxman MN. Clinical manifestations of herpes zoster. In : Arvin AM, Gershon AA, editors. Varicella-zoster virus: virology and clinical management. Cambridge: Cambridge University Press;2000. p. 246.2. Levin MJ, Smith JG, Kaufhold RM, Barber D, Hayward AR, Chan CY, Chan IS, Li DJ, Wang W, Keller PM, Shaw A, Silber JL, Schlienger K, Chalikonda I, Vessey SJ, Caulfield MJ. Decline in varicella-zoster virus (VZV)-specific cell-mediated immunity with increasing age and boosting with a high-dose VZV vaccine. J Infect Dis. 2003; 188:1336–1344.
Article3. Kawai K, Yawn BP. Risk factors for herpes zoster: a systematic review and meta-analysis. Mayo Clin Proc. 2017; 92:1806–1821.
Article4. Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014; 4:e004833.
Article5. Takao Y, Miyazaki Y, Okeda M, Onishi F, Yano S, Gomi Y, Ishikawa T, Okuno Y, Mori Y, Asada H, Yamanishi K, Iso H. Incidences of herpes zoster and postherpetic neuralgia in japanese adults aged 50 years and older from a community-based prospective cohort study: the SHEZ study. J Epidemiol. 2015; 25:617–625.
Article6. Kim YJ, Lee CN, Lim CY, Jeon WS, Park YM. Population-based study of the epidemiology of herpes zoster in Korea. J Korean Med Sci. 2014; 29:1706–1710.
Article7. Schmader K, Gnann JW Jr, Watson CP. The epidemiological, clinical, and pathological rationale for the herpes zoster vaccine. J Infect Dis. 2008; 197:Suppl 2. S207–S215.
Article8. Thomas SL, Hall AJ. What does epidemiology tell us about risk factors for herpes zoster? Lancet Infect Dis. 2004; 4:26–33.
Article9. Yawn BP, Wollan PC, Kurland MJ, St Sauver JL, Saddier P. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc. 2011; 86:88–93.
Article10. Shiraki K, Toyama N, Daikoku T, Yajima M. Miyazaki Dermatologist Society. Herpes zoster and recurrent herpes zoster. Open Forum Infect Dis. 2017; 4:ofx007.
Article11. Kalman CM, Laskin OL. Herpes zoster and zosteriform herpes simplex virus infections in immunocompetent adults. Am J Med. 1986; 81:775–778.
Article12. Dayan RR, Peleg R. Herpes zoster: typical and atypical presentations. Postgrad Med. 2017; 129:567–571.13. Hope-simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med. 1965; 58:9–20.
Article14. Lopez AS, Burnett-Hartman A, Nambiar R, Ritz L, Owens P, Loparev VN, Guris D, Schmid DS. Transmission of a newly characterized strain of varicella-zoster virus from a patient with herpes zoster in a long-term-care facility, West Virginia, 2004. J Infect Dis. 2008; 197:646–653.
Article15. Marin M, Guris D, Chaves SS, Schmid S, Seward JF. Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007; 56(RR-4):1–40.16. Tyring SK. Management of herpes zoster and postherpetic neuralgia. J Am Acad Dermatol. 2007; 57:6 Suppl. S136–S142.
Article17. John AR, Canaday DH. Herpes zoster in the older adult. Infect Dis Clin North Am. 2017; 31:811–826.
Article18. Kang JH, Ho JD, Chen YH, Lin HC. Increased risk of stroke after a herpes zoster attack: a population-based follow-up study. Stroke. 2009; 40:3443–3448.
Article19. Kim MC, Yun SC, Lee HB, Lee PH, Lee SW, Choi SH, Kim YS, Woo JH, Kim SH, Kwon SU. Herpes zoster increases the risk of stroke and myocardial infarction. J Am Coll Cardiol. 2017; 70:295–296.
Article20. Cohen JI. Clinical practice: herpes zoster. N Engl J Med. 2013; 369:255–263.21. Bader MS. Herpes zoster: diagnostic, therapeutic, and preventive approaches. Postgrad Med. 2013; 125:78–91.
Article22. Breuer J, Whitley R. Varicella zoster virus: natural history and current therapies of varicella and herpes zoster. Herpes. 2007; 14:Suppl 2. 25–29.23. Baron R. Post-herpetic neuralgia case study: optimizing pain control. Eur J Neurol. 2004; 11:Suppl 1. 3–11.
Article24. Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, Weinberg A, Boardman KD, Williams HM, Zhang JH, Peduzzi PN, Beisel CE, Morrison VA, Guatelli JC, Brooks PA, Kauffman CA, Pachucki CT, Neuzil KM, Betts RF, Wright PF, Griffin MR, Brunell P, Soto NE, Marques AR, Keay SK, Goodman RP, Cotton DJ, Gnann JW Jr, Loutit J, Holodniy M, Keitel WA, Crawford GE, Yeh SS, Lobo Z, Toney JF, Greenberg RN, Keller PM, Harbecke R, Hayward AR, Irwin MR, Kyriakides TC, Chan CY, Chan IS, Wang WW, Annunziato PW, Silber JL. Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005; 352:2271–2284.
Article25. Harpaz R, Ortega-Sanchez IR, Seward JF. Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2008; 57(RR-5):1–30.26. Schmader KE, Levin MJ, Gnann JW Jr, McNeil SA, Vesikari T, Betts RF, Keay S, Stek JE, Bundick ND, Su SC, Zhao Y, Li X, Chan IS, Annunziato PW, Parrino J. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50–59 years. Clin Infect Dis. 2012; 54:922–928.
Article27. Wyman MJ, Stabi KL. Concomitant administration of pneumococcal-23 and zoster vaccines provides adequate herpes zoster coverage. Ann Pharmacother. 2013; 47:1064–1068.
Article28. Mills R, Tyring SK, Levin MJ, Parrino J, Li X, Coll KE, Stek JE, Schlienger K, Chan IS, Silber JL. Safety, tolerability, and immunogenicity of zoster vaccine in subjects with a history of herpes zoster. Vaccine. 2010; 28:4204–4209.
Article29. Weinberg A, Zhang JH, Oxman MN, Johnson GR, Hayward AR, Caulfield MJ, Irwin MR, Clair J, Smith JG, Stanley H, Marchese RD, Harbecke R, Williams HM, Chan IS, Arbeit RD, Gershon AA, Schödel F, Morrison VA, Kauffman CA, Straus SE, Schmader KE, Davis LE, Levin MJ. US Department of Veterans Affairs (VA) Cooperative Studies Program Shingles Prevention Study Investigators. Varicella-zoster virus-specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. J Infect Dis. 2009; 200:1068–1077.
Article30. Tseng HF, Harpaz R, Luo Y, Hales CM, Sy LS, Tartof SY, Bialek S, Hechter RC, Jacobsen SJ. Declining effectiveness of herpes zoster vaccine in adults aged ≥60 years. J Infect Dis. 2016; 213:1872–1875.
Article31. Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang SJ, Díez-Domingo J, Godeaux O, Levin MJ, McElhaney JE, Puig-Barbera J, Vanden Abeele C, Vesikari T, Watanabe D, Zahaf T, Ahonen A, Athan E, Barba-Gomez JF, Campora L, de Looze F, Downey HJ, Ghesquiere W, Gorfinkel I, Korhonen T, Leung E, McNeil SA, Oostvogels L, Rombo L, Smetana J, Weckx L, Yeo W, Heineman TC. ZOE-70 Study Group. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016; 375:1019–1032.
Article32. Le P, Rothberg MB. Cost-effectiveness of herpes zoster vaccine for persons aged 50 years. Ann Intern Med. 2015; 163:489–497.
Article33. Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ, Levin MJ, McElhaney JE, Poder A, Puig-Barbera J, Vesikari T, Watanabe D, Weckx L, Zahaf T, Heineman TC. ZOE-50 Study Group. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015; 372:2087–2096.
Article