Lab Anim Res.
2017 Jun;33(2):165-170. 10.5625/lar.2017.33.2.165.
Comparision of doxorubicin-induced cardiotoxicity in the ICR mice of different sources
- Affiliations
-
- 1College of Pharmacy, Pusan National University, Busan, Korea. youngjung@pusan.ac.kr
- 2Department of Biomedical Laboratory Science, Daekyeung College, Gyeongsan, Korea.
- 3College of Pharmacy, Kyungsung University, Busan, Korea.
- 4Department of Microbiology and Immunology, INJE University College of Medicine, Busan, Korea.
- 5Exercise Biochemistry Laboratory, Korea National Sport University, Seoul, Korea.
- 6Department of Biomaterials Science, College of Natural Resources & Life Science/Life and Industry Convergence Research Institute, Pusan National University, Miryang, Korea.
- 7College of Veterinary Medicine, Kyungpook National University, Daegu, Korea.
- KMID: 2407431
- DOI: http://doi.org/10.5625/lar.2017.33.2.165
Abstract
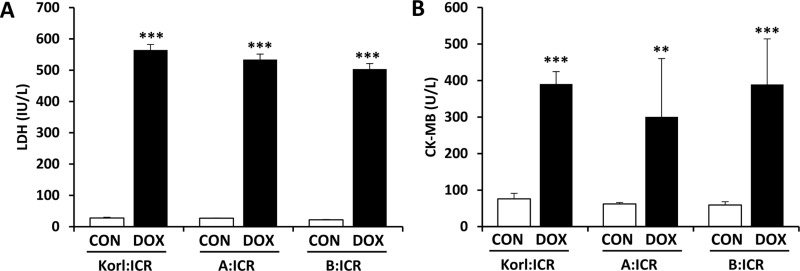
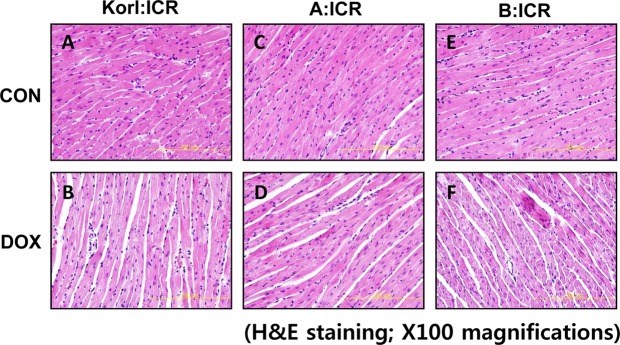
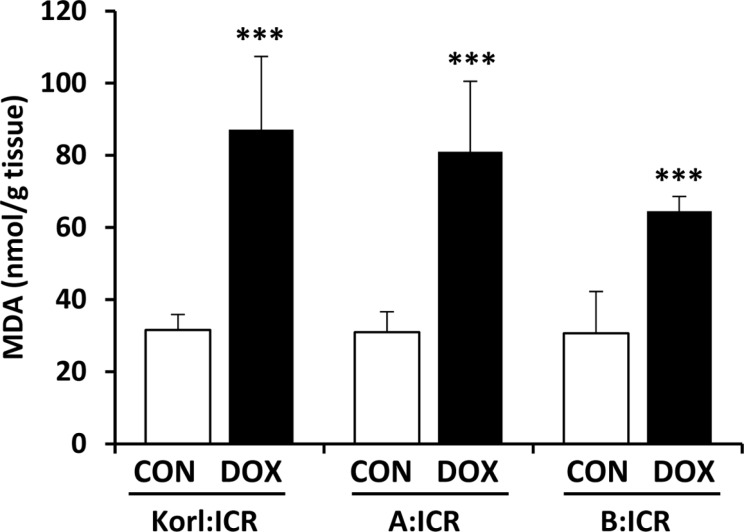
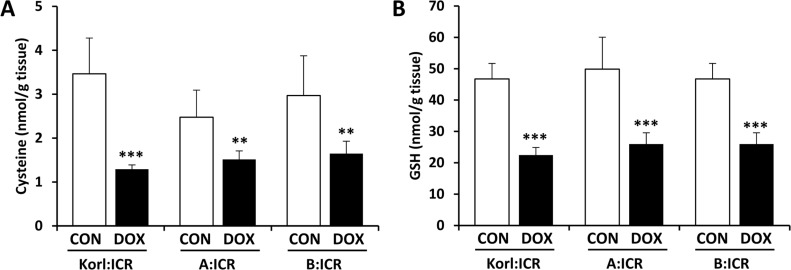
- Doxorubicin is a widely used chemotherapeutic agents and is now part of standard therapeutic regimens for a variety of cancers (eg, hematopoietic malignancies and advanced solid tumors of the breast, ovary, thyroid, and bone). However, a potentially lethal and dose-dependent cardiotoxicity that appears within a short time after treatment limits the usage of doxorubicin in cancer patients. Although the mechanism of doxorubicin-induced cardiotoxicity is not completely understood, it is thought that free radical-induced oxidative stress and excessive production of reactive oxygen species are primary drivers of its toxicity. In this study, we compared the doxorubicin-induced cardiotoxicity of ICR mice obtained from three different sources and evaluated the utility of Korl:ICR stock established by the Korean FDA. Because doxorubicin-induced cardiotoxicity is thought to involve the excessive generation of ROS followed by oxidative stress, we determined the representative tissue index of oxidation, lipid peroxidation, and antioxidant, glutathione (GSH), as well as the parameters of heart injury. Doxorubicin treatment successfully induced cardiotoxicity as evidenced by histological examination and serum parameters (eg, levels of LDH and CK activities) in ICR mice. It was accompanied by increased lipid peroxidation and a decrease in both cysteine and GSH, further supporting previous reports that oxidative stress is a potential mechanism of doxorubicin-induced cardiotoxicity. Of interest, we did not observe a significant difference in doxorubicin-induced cardiotoxicity among mice of different origins. Collectively, our results suggest that Korl:ICR strain may be useful in the research of doxorubicin-induced cardiotoxicity.
Keyword
MeSH Terms
Figure
Reference
-
1. Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014; 63(25 Pt A):2751–2768. PMID: 24703918.2. Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004; 56(2):185–229. PMID: 15169927.
Article3. Perrino C, Schiattarella GG, Magliulo F, Ilardi F, Carotenuto G, Gargiulo G, Serino F, Ferrone M, Scudiero F, Carbone A, Trimarco B, Esposito G. Cardiac side effects of chemotherapy: state of art and strategies for a correct management. Curr Vasc Pharmacol. 2014; 12(1):106–116. PMID: 22563720.
Article4. Raschi E, Vasina V, Ursino MG, Boriani G, Martoni A, De Ponti F. Anticancer drugs and cardiotoxicity: Insights and perspectives in the era of targeted therapy. Pharmacol Ther. 2010; 125(2):196–218. PMID: 19874849.
Article5. Carter SK. Adriamycin-Review. J Natl Cancer Inst. 1975; 55(6):1265–1274. PMID: 1107570.6. Bristow MR, Mason JW, Billingham ME, Daniels JR. Doxorubicin Cardiomyopathy-Evaluation by Phonocardiography, Endomyocardial Biopsy, and Cardiac-Catheterization. Ann Intern Med. 1978; 88(2):168–175. PMID: 626445.7. Bristow MR, Thompson PD, Martin RP, Mason JW, Billingham ME, Harrison DC. Early Anthracycline Cardiotoxicity. Am J Med. 1978; 65(5):823–832. PMID: 707541.
Article8. Maksimenko AV, Vavaev AV. Antioxidant enzymes as potential targets in cardioprotection and treatment of cardiovascular diseases. Enzyme antioxidants: the next stage of pharmacological counterwork to the oxidative stress. Heart Int. 2012; 7(1):e3. PMID: 22690296.
Article9. Todorova VK, Beggs ML, Delongchamp RR, Dhakal I, Makhoul I, Wei JY, Klimberg VS. Transcriptome profiling of peripheral blood cells identifies potential biomarkers for doxorubicin cardiotoxicity in a rat model. PLoS One. 2012; 7(11):e48398. PMID: 23209553.
Article10. Sterba M, Popelova O, Vavrova A, Jirkovsky E, Kovarikova P, Gersl V, Simunek T. Oxidative stress, redox signaling, and metal chelation in anthracycline cardiotoxicity and pharmacological cardioprotection. Antioxid Redox Signal. 2013; 18(8):899–929. PMID: 22794198.11. Thayer WS. Adriamycin Stimulated Superoxide Formation in Sub-Mitochondrial Particles. Chem Biol Interact. 1977; 19(3):265–278. PMID: 202411.
Article12. Doroshow JH, Reeves J. Anthracycline-Enhanced Oxygen Radical Formation in the Heart. Proc Am Assoc Cancer Res. 1980; 21:266.13. Carvalho FS, Burgeiro A, Garcia R, Moreno AJ, Carvalho RA, Oliveira PJ. Doxorubicin-induced cardiotoxicity: from bioenergetic failure and cell death to cardiomyopathy. Med Res Rev. 2014; 34(1):106–135. PMID: 23494977.
Article14. Chia R, Achilli F, Festing MF, Fisher EM. The origins and uses of mouse outbred stocks. Nat Genet. 2005; 37(11):1181–1186. PMID: 16254564.
Article15. Nolin TD, McMenamin ME, Himmelfarb J. Simultaneous determination of total homocysteine, cysteine, cysteinylglycine, and glutathione in human plasma by high-performance liquid chromatography: application to studies of oxidative stress. J Chromatogr B Analyt Technol Biomed Life Sci. 2007; 852(1-2):554–561.
Article16. Myers CE, McGuire WP, Liss RH, Ifrim I, Grotzinger K, Young RC. Adriamycin: the role of lipid peroxidation in cardiac toxicity and tumor response. Science. 1977; 197(4299):165–167. PMID: 877547.
Article17. Lu SC. Glutathione synthesis. Biochim Biophys Acta. 2013; 1830(5):3143–3153. PMID: 22995213.
Article18. Jung YS, Kim SJ, Kwon DY, Kim YC. Comparison of the effects of buthioninesulfoximine and phorone on the metabolism of sulfur-containing amino acids in rat liver. Biochem Biophys Res Commun. 2008; 368(4):913–918. PMID: 18275846.
Article19. Doroshow JH, Locker GY, Baldinger J, Myers CE. The effect of doxorubicin on hepatic and cardiac glutathione. Res Commun Chem Pathol Pharmacol. 1979; 26(2):285–295. PMID: 574979.20. Indu R, Azhar TS, Nair A, Nair CK. Amelioration of doxorubicin induced cardio-and hepato-toxicity by carotenoids. J Cancer Res Ther. 2014; 10(1):62–67. PMID: 24762488.21. Shin HJ, Cho YM, Shin HJ, Kim HD, Choi KM, Kim MG, Shin HD, Chung MW. Comparison of commonly used ICR stocks and the characterization of Korl:ICR. Lab Anim Res. 2017; 33(1):8–14. PMID: 28400834.
Article22. Tan G, Lou Z, Liao W, Zhu Z, Dong X, Zhang W, Li W, Chai Y. Potential biomarkers in mouse myocardium of doxorubicin-induced cardiomyopathy: a metabonomic method and its application. PLoS One. 2011; 6(11):e27683. PMID: 22110719.
Article23. Liu X, Chen Z, Chua CC, Ma YS, Youngberg GA, Hamdy R, Chua BH. Melatonin as an effective protector against doxorubicin-induced cardiotoxicity. Am J Physiol Heart Circ Physiol. 2002; 283(1):H254–H263. PMID: 12063298.24. Fisher PW, Salloum F, Das A, Hyder H, Kukreja RC. Phosphodiesterase-5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation. 2005; 111(13):1601–1610. PMID: 15811867.
Article25. Ma Y, Zhang X, Bao H, Mi S, Cai W, Yan H, Wang Q, Wang Z, Yan J, Fan GC. Toll-like receptor (TLR) 2 and TLR4 differentially regulate doxorubicin induced cardiomyopathy in mice. PLoS One. 2012; 7(7):e40763. PMID: 22808256.
Article26. Thayer WS. Adriamycin stimulated superoxide formation in submitochondrial particles. Chem Biol Interact. 1977; 19(3):265–278. PMID: 202411.
Article27. Doroshow JH, Locker GY, Ifrim I, Myers CE. Prevention of doxorubicin cardiac toxicity in the mouse by N-acetylcysteine. J Clin Invest. 1981; 68(4):1053–1064. PMID: 7287901.
Article28. Doroshow JH, Locker GY, Myers CE. Enzymatic defenses of the mouse heart against reactive oxygen metabolites: alterations produced by doxorubicin. J Clin Invest. 1980; 65(1):128–135. PMID: 7350193.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pathophysiology and preventive strategies of anthracycline-induced cardiotoxicity
- Comparison of the response using ICR mice derived from three different sources to multiple low-dose streptozotocin-induced diabetes mellitus
- Loss of endogenous estrogen increases cardiac toxicity by doxorubicin
- Evaluation of Left Ventricular Diastolic Function in Patients Receiving Doxorubicin
- Doxorubicin Cardiotoxicity: Response of Left Ventricular Ejection Fraction to Exercise and Incidence of Regional Wall Motion Abnormalities