Endocrinol Metab.
2015 Sep;30(3):371-380. 10.3803/EnM.2015.30.3.371.
Physiological Parameters in the Blood of a Murine Stress-Induced Depression Model before and after Repeated Passive Exercise
- Affiliations
-
- 1Department of Brain and Cognitive Sciences, Ewha Womans University, Seoul, Korea. plhan@ewha.ac.kr
- 2Department of Chemistry and Nano Science, Ewha Womans University, Seoul, Korea.
- 3Brain Disease Research Institute, Ewha Womans University, Seoul, Korea.
- KMID: 2407089
- DOI: http://doi.org/10.3803/EnM.2015.30.3.371
Abstract
- BACKGROUND
Animal models are necessary to study the mechanism underlying the effects of exercise on depression but an effective procedure for exercise treatment and exercise effects on physiological parameters in a specific depression model need to be characterized.
METHODS
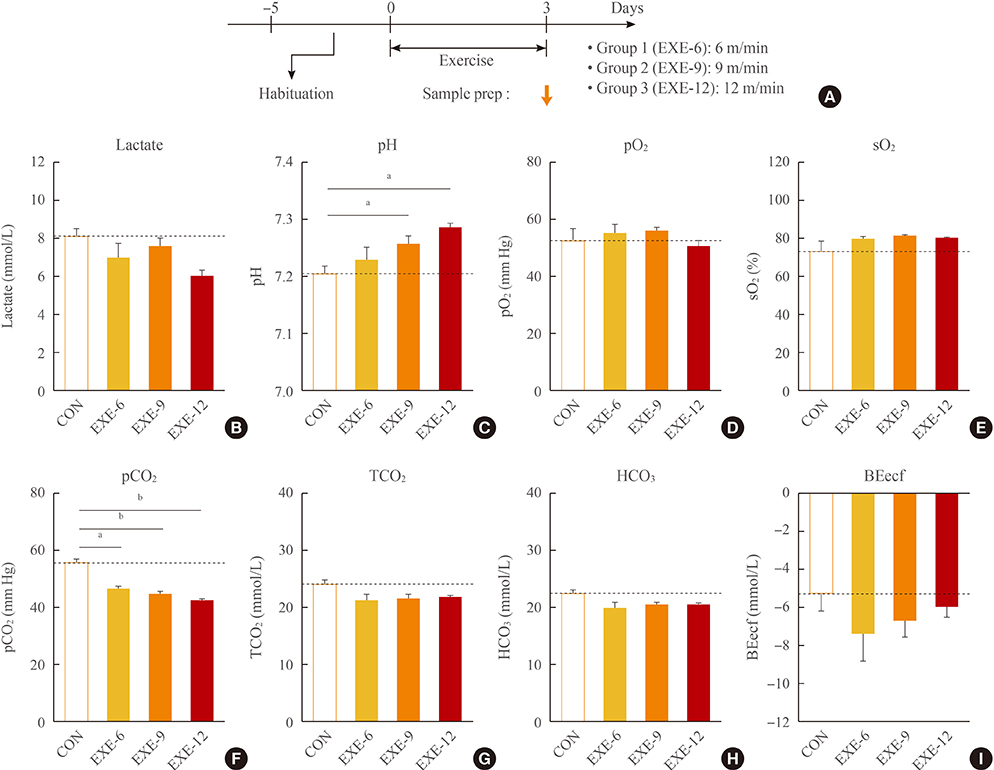
Physiological parameters including lactate, partial pressue of O2 (pO2) and CO2 (pCO2) saturated O2 (sO2), pH, HCO3, total CO2 (TCO2), and base excess extracellular fluid (BEecf) levels in the blood were measured after treatment with passive exercise in normal mice and a stress-induced depression model.
RESULTS
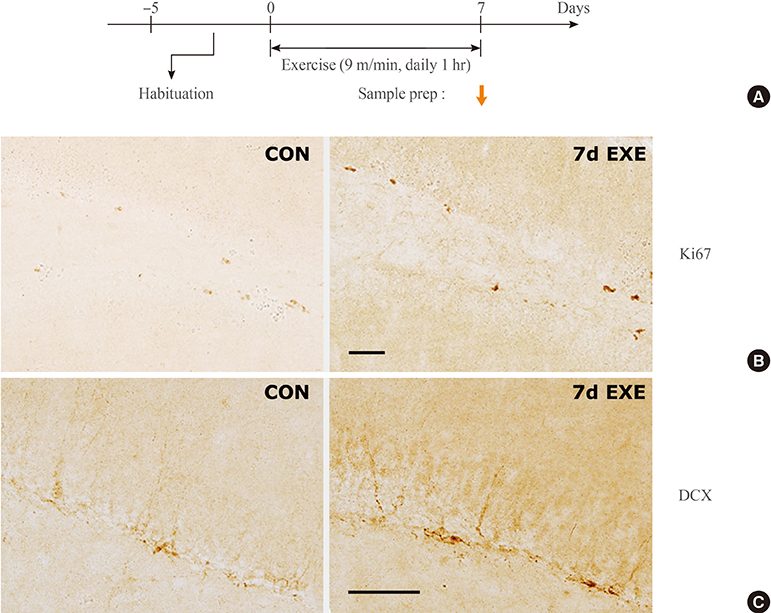
Normal mice or mice that were subjected to daily 2-hour restraint for 14 days (2 hoursx14 days of restraint) were placed on a running wheel that was rotating at a speed of 9 m/min for 1 hour per day for 1 to 21 days. After repeated exercise in mice that were previously subjected to 2 hoursx14 days restraint, plasma lactate levels decreased, the levels of pO2, sO2, and pH tended to increase, and the levels of pCO2 decreased in the absence of significant changes in HCO3, TCO2, and BEecf. However, none of these changes were additive to the stress effects or were much more severe than those induced after repeated passive exercise in normal mice.
CONCLUSION
These results suggest that passive exercise for 1 hour daily for 14 to 21 consecutive days on a running wheel rotating at a speed of 9 m/min may be used as an exercise protocol without inducing severe additive effects on physiological burdens.
MeSH Terms
Figure
Reference
-
1. World Health Organization. Search results 'depression' 2014 [Internet]. Geneva: WHO;c2013. cited 2014 Dec 29. Available from: http://search.who.int/search?q=depression&ie=utf8&site=who&client=_en_r&proxystylesheet=_en_r&output=xml_no_dtd&oe=utf8&getfields=doctype.2. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Washington, DC: American Psychiatric Association;2013.3. Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008; 455:894–902.4. Banasr M, Valentine GW, Li XY, Gourley SL, Taylor JR, Duman RS. Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biol Psychiatry. 2007; 62:496–504.5. Banasr M, Duman RS. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol Psychiatry. 2008; 64:863–870.6. Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011; 69:754–761.7. Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006; 311:864–868.8. Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007; 131:391–404.9. Kim KS, Han PL. Optimization of chronic stress paradigms using anxiety- and depression-like behavioral parameters. J Neurosci Res. 2006; 83:497–507.10. Seo JS, Park JY, Choi J, Kim TK, Shin JH, Lee JK, et al. NADPH oxidase mediates depressive behavior induced by chronic stress in mice. J Neurosci. 2012; 32:9690–9699.11. Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2012; 39:112–119.12. Leasure JL, Jones M. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience. 2008; 156:456–465.13. Cook MD, Martin SA, Williams C, Whitlock K, Wallig MA, Pence BD, et al. Forced treadmill exercise training exacerbates inflammation and causes mortality while voluntary wheel training is protective in a mouse model of colitis. Brain Behav Immun. 2013; 33:46–56.14. Liu YF, Chen HI, Wu CL, Kuo YM, Yu L, Huang AM, et al. Differential effects of treadmill running and wheel running on spatial or aversive learning and memory: roles of amygdalar brain-derived neurotrophic factor and synaptotagmin I. J Physiol. 2009; 587(Pt 13):3221–3231.15. Milne KJ, Noble EG. Exercise-induced elevation of HSP70 is intensity dependent. J Appl Physiol (1985). 2002; 93:561–568.16. Pires FO, Noakes TD, Lima-Silva AE, Bertuzzi R, Ugrinowitsch C, Lira FS, et al. Cardiopulmonary, blood metabolite and rating of perceived exertion responses to constant exercises performed at different intensities until exhaustion. Br J Sports Med. 2011; 45:1119–1125.17. Garcia TR, Carneiro de Rezende AS, Santiago JM, Queiroz de Almeida F, Fonseca MG, Munoz A. Venous hemogasometry and blood electrolytes in mangalarga marchador mares submitted to aerobic training. Ciênc Agrotec. 2013; 37:559–565.18. Brown ET, Umino Y, Loi T, Solessio E, Barlow R. Anesthesia can cause sustained hyperglycemia in C57/BL6J mice. Vis Neurosci. 2005; 22:615–618.19. Gjedde A, Rasmussen M. Pentobarbital anesthesia reduces blood-brain glucose transfer in the rat. J Neurochem. 1980; 35:1382–1387.20. Baek IS, Kim TK, Seo JS, Lee KW, Lee YA, Cho J, et al. AAD-2004 attenuates progressive neuronal loss in the brain of Tg-betaCTF99/B6 mouse model of Alzheimer disease. Exp Neurobiol. 2013; 22:31–37.21. Silverman SC, Birks EK. Evaluation of the i-STAT hand-held chemical analyser during treadmill and endurance exercise. Equine Vet J Suppl. 2002; 551–554.22. Tinkey P, Lembo T, Craig S, West C, Van Pelt C. Use of the i-STAT portable clinical analyzer in mice. Lab Anim (NY). 2006; 35:45–50.23. Dascombe BJ, Reaburn PR, Sirotic AC, Coutts AJ. The reliability of the i-STAT clinical portable analyser. J Sci Med Sport. 2007; 10:135–140.24. Park JY, Kim TK, Choi J, Lee JE, Kim H, Lee EH, et al. Implementation of a two-dimensional behavior matrix to distinguish individuals with differential depression states in a rodent model of depression. Exp Neurobiol. 2014; 23:215–223.25. Wisloff U, Helgerud J, Kemi OJ, Ellingsen O. Intensity-controlled treadmill running in rats: VO(2 max) and cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2001; 280:H1301–H1310.26. Kemi OJ, Loennechen JP, Wisloff U, Ellingsen O. Intensity-controlled treadmill running in mice: cardiac and skeletal muscle hypertrophy. J Appl Physiol (1985). 2002; 93:1301–1309.27. Hoydal MA, Wisloff U, Kemi OJ, Ellingsen O. Running speed and maximal oxygen uptake in rats and mice: practical implications for exercise training. Eur J Cardiovasc Prev Rehabil. 2007; 14:753–760.28. Aguiar AS Jr, Boemer G, Rial D, Cordova FM, Mancini G, Walz R, et al. High-intensity physical exercise disrupts implicit memory in mice: involvement of the striatal glutathione antioxidant system and intracellular signaling. Neuroscience. 2010; 171:1216–1227.29. Leem YH, Lim HJ, Shim SB, Cho JY, Kim BS, Han PL. Repression of tau hyperphosphorylation by chronic endurance exercise in aged transgenic mouse model of tauopathies. J Neurosci Res. 2009; 87:2561–2570.30. Um HS, Kang EB, Koo JH, Kim HT, Jin L, Kim EJ, et al. Treadmill exercise represses neuronal cell death in an aged transgenic mouse model of Alzheimer's disease. Neurosci Res. 2011; 69:161–173.31. Nam SM, Kim JW, Yoo DY, Choi JH, Kim W, Jung HY, et al. Effects of treadmill exercise on neural stem cells, cell proliferation, and neuroblast differentiation in the subgranular zone of the dentate gyrus in cyclooxygenase-2 knockout mice. Neurochem Res. 2013; 38:2559–2569.32. Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008; 155:1048–1058.33. Van der Borght K, Kobor-Nyakas DE, Klauke K, Eggen BJ, Nyakas C, Van der Zee EA, et al. Physical exercise leads to rapid adaptations in hippocampal vasculature: temporal dynamics and relationship to cell proliferation and neurogenesis. Hippocampus. 2009; 19:928–936.34. Koehl M, Meerlo P, Gonzales D, Rontal A, Turek FW, Abrous DN. Exercise-induced promotion of hippocampal cell proliferation requires beta-endorphin. FASEB J. 2008; 22:2253–2262.35. Fuss J, Ben Abdallah NM, Vogt MA, Touma C, Pacifici PG, Palme R, et al. Voluntary exercise induces anxiety-like behavior in adult C57BL/6J mice correlating with hippocampal neurogenesis. Hippocampus. 2010; 20:364–376.36. Duman CH, Schlesinger L, Russell DS, Duman RS. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008; 1199:148–158.37. Salam JN, Fox JH, Detroy EM, Guignon MH, Wohl DF, Falls WA. Voluntary exercise in C57 mice is anxiolytic across several measures of anxiety. Behav Brain Res. 2009; 197:31–40.38. Snyder JS, Glover LR, Sanzone KM, Kamhi JF, Cameron HA. The effects of exercise and stress on the survival and maturation of adult-generated granule cells. Hippocampus. 2009; 19:898–906.39. Kannangara TS, Lucero MJ, Gil-Mohapel J, Drapala RJ, Simpson JM, Christie BR, et al. Running reduces stress and enhances cell genesis in aged mice. Neurobiol Aging. 2011; 32:2279–2286.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A novel biomarker of exercise-induced stress in horses
- Stress-Induced Depression Is Alleviated by Aerobic Exercise Through Up-Regulation of 5-Hydroxytryptamine 1A Receptors in Rats
- Effects of Physical Activity and Melatonin in a Rat Model of Depression Induced by Chronic Stress
- The effects of regular exercise on capsaicin-induced pulpal pain and pain-induced changes in passive avoidance learning and memory in rats
- The Effect of the Walking Exercise on Physiological index, Physical Fitness, Self Esteem, Depression and Life Satisfaction in the Institutionalized Elderly Women