J Korean Neurosurg Soc.
2018 Jan;61(1):81-88. 10.3340/jkns.2016.1212.005.
Comparative Analysis of Spontaneous Infectious Spondylitis : Pyogenic versus Tuberculous
- Affiliations
-
- 1Department of Neurosurgery, Korea University Ansan Hospital, Ansan, Korea. nsbjkim@gmail.com
- KMID: 2403527
- DOI: http://doi.org/10.3340/jkns.2016.1212.005
Abstract
OBJECTIVE
Spondylitis is often chemotherapy resistant and requires long-term treatment. Without adequate chemotherapy, the outcome can be fatal or result in severe neurologic damage. Therefore, differentiating the etiology of spondylitis is very important, particularly in spontaneous cases. As the prevalence of tuberculosis in Korea has decreased in recent years, updated clinical research about spondylitis is warranted.
METHODS
From April 2010 to March 2016, data from spondylitis patients were collected retrospectively. In total, 69 patients (51 with pyogenic spondylitis and 18 with tuberculous spondylitis) were included. Clinical data, laboratory findings including erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level, measurements of Cobb angles at the initial and final follow-up, and radiologic features on magnetic resonance imaging (MRI) scans were evaluated. To test differences between the pyogenic and tuberculous groups, numerical data were compared using the student's t-test and Mann-Whitney U test, and categorical data were compared using the chi-square test and Fisher's exact test.
RESULTS
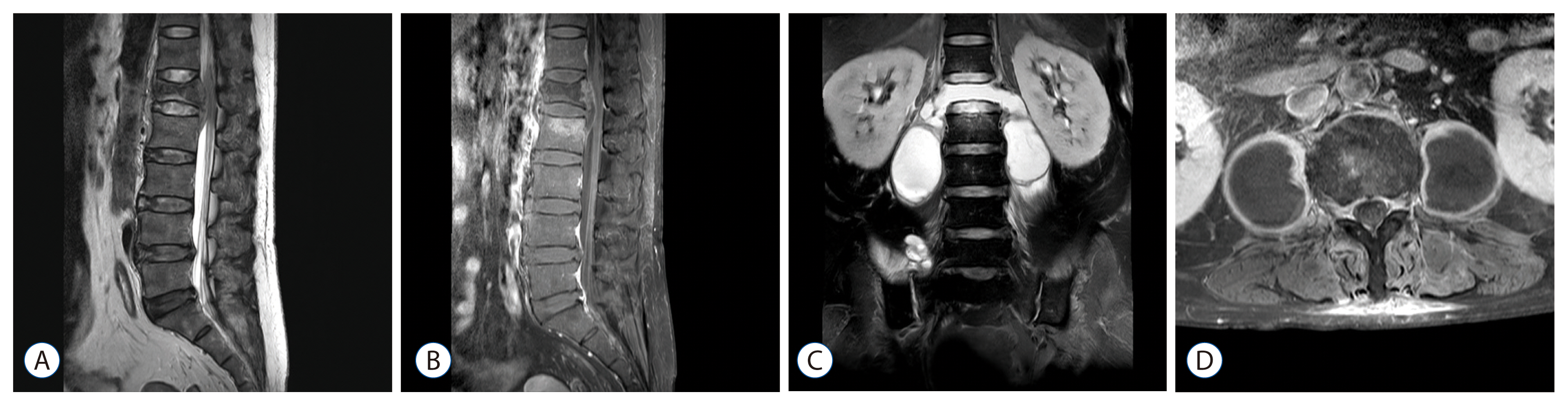
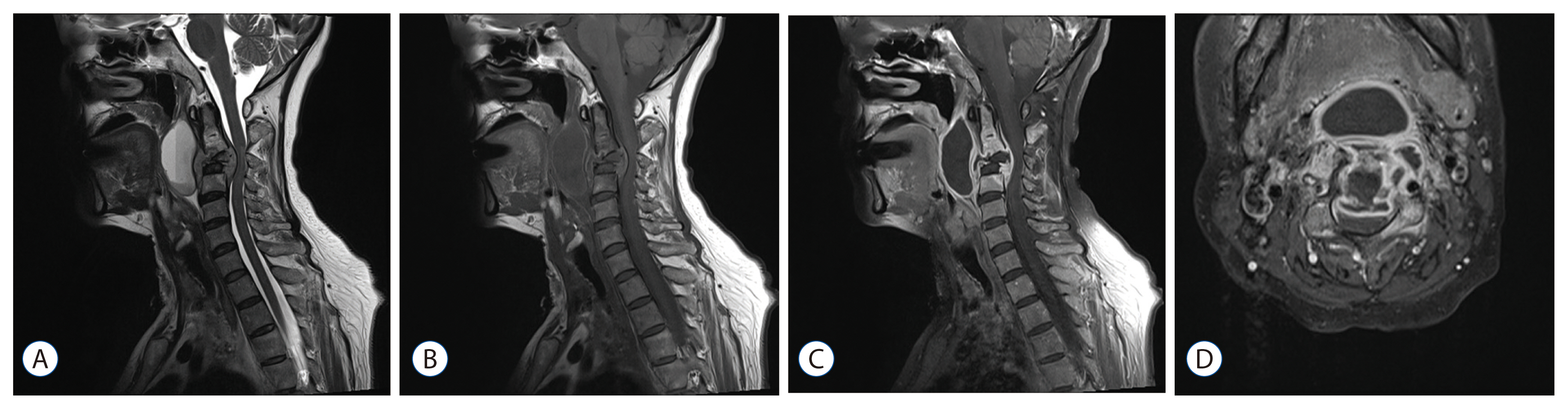
The patients' mean age was 60.0 years. Male sex was slightly predominant (56.5%). There was no difference in mean age and sex between the two groups. The pyogenic group had a relatively higher proportion of immunocompromised patients. The peak CRP value was higher in the pyogenic group than in the tuberculous group (14.08 mg/dL and 8.50 mg/dL, respectively, p=0.009), whereas the ESR was not significantly different between the groups (81.5 mm/h and 75.6 mm/h, respectively, p=0.442). Radiologically, the presence of disc space sparing and vertebral body collapse differed between the groups. In the tuberculous group, the disc was more commonly preserved on contrast-enhanced MRI (50% and 23.5%, respectively, p=0.044), and vertebral body collapse was more common (66.6% and 15.7%, respectively, p < 0.001). The mean length of hospitalization was longer in the pyogenic group (56.5 days and 41.2 days, respectively, p=0.001). Four mortality cases were observed only in the pyogenic group. The most commonly isolated microorganism in the pyogenic group was Staphylococcus aureus (S. aureus) (methicillin susceptible S. aureus and methicillin resistant S. aureus [MRSA] in 8 and 4 cases, respectively).
CONCLUSION
The clinical and radiological manifestations of spontaneous spondylitis differ based on the causative organism. Pyogenic spondylitis patients tend to have a higher CRP level and a more severe clinical course, whereas tuberculous spondylitis patients present with destruction of the vertebral body with disc sparing more frequently. The presence of MRSA is increasing in community-acquired spondylitis cases.
Keyword
MeSH Terms
-
Bacterial Infections
Blood Sedimentation
Bone Diseases, Infectious
C-Reactive Protein
Discitis
Drug Therapy
Follow-Up Studies
Hospitalization
Humans
Immunocompromised Host
Korea
Magnetic Resonance Imaging
Male
Methicillin Resistance
Methicillin-Resistant Staphylococcus aureus
Mortality
Osteomyelitis
Prevalence
Retrospective Studies
Spondylitis*
Staphylococcus aureus
Tuberculosis
C-Reactive Protein
Figure
Reference
-
References
1. An HS, Seldomridge JA. Spinal infections: diagnostic tests and imaging studies. Clin Orthop Relat Res. 444:27–33. 2006.2. Berbari EF, Kanj SS, Kowalski TJ, Darouiche RO, Widmer AF, Schmitt SK, et al. Executive summary: 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis. 61:859–863. 2015.
Article3. Chang MC, Wu HT, Lee CH, Liu CL, Chen TH. Tuberculous spondylitis and pyogenic spondylitis: comparative magnetic resonance imaging features. Spine (Phila Pa 1976). 31:782–788. 2006.4. Cheung W, Luk KD. Pyogenic spondylitis. Int Orthop. 36:397–404. 2012.
Article5. Colmenero JD, Jiménez-Mejías ME, Sánchez-Lora FJ, Reguera JM, Palomino-Nicás J, Martos F, et al. Pyogenic, tuberculous, and brucellar vertebral osteomyelitis: a descriptive and comparative study of 219 cases. Ann Rheum Dis. 56:709–715. 1997.
Article6. Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 284:1318–1322. 1999.
Article7. Cottle L, Riordan T. Infectious spondylodiscitis. J Infect. 56:401–412. 2008.
Article8. De Backer AI, Mortelé KJ, Vanschoubroeck IJ, Deeren D, Vanhoenacker FM, De Keulenaer BL, et al. Tuberculosis of the spine: CT and MR imaging features. JBR-BTR. 88:92–97. 2005.9. Donlan RM. Biofilms and device-associated infections. Emerg Infect Dis. 7:277–281. 2001.
Article10. Høiby N, Bjarnsholt T, Moser C, Bassi GL, Coenye T, Donelli G, et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect. 21:Suppl 1. S1–S25. 2015.
Article11. Ha KY, Chung YG, Ryoo SJ. Adherence and biofilm formation of staphylococcus epidermidis and mycobacterium tuberculosis on various spinal implants. Spine (Phila Pa 1976). 30:38–43. 2005.
Article12. Hong YP, Kim SJ, Lew WJ, Lee EK, Han YC. The seventh nationwide tuberculosis prevalence survey in Korea, 1995. Int J Tuberc Lung Dis. 2:27–36. 1998.13. Jeong SJ, Choi SW, Youm JY, Kim HW, Ha HG, Yi JS. Microbiology and epidemiology of infectious spinal disease. J Korean Neurosurg Soc. 56:21–27. 2014.
Article14. Jevtic V. Vertebral infection. Eur Radiol. 14:Suppl 3. E43–E52. 2004.
Article15. Joseffer SS, Cooper PR. Modern imaging of spinal tuberculosis. J Neurosurg Spine. 2:145–150. 2005.
Article16. Jung NY, Jee WH, Ha KY, Park CK, Byun JY. Discrimination of tuberculous spondylitis from pyogenic spondylitis on MRI. AJR Am J Roentgenol. 182:1405–1410. 2004.
Article17. Kim CJ, Song KH, Jeon JH, Park WB, Park SW, Kim HB, et al. A comparative study of pyogenic and tuberculous spondylodiscitis. Spine (Phila Pa 1976). 35:E1096–E1100. 2010.
Article18. Kim HJ. Current status of tuberculosis in Korea. Korean J Med. 82:257–262. 2011.
Article19. Korean Society for Chemotherapy, Korean Society of Infectious Diseases, Korean Orthopaedic Association. Clinical guidelines for the antimicrobial treatment of bone and joint infections in Korea. Infect Chemother. 46:125–138. 2014.20. Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 364:369–379. 2004.
Article21. Nahid P, Dorman SE, Alipanah N, Barry PM, Brozek JL, Cattamanchi A, et al. Official American thoracic society/centers for disease control and prevention/infectious diseases society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 63:e147–e195. 2016.22. Pigrau-Serrallach C, Rodríguez-Pardo D. Bone and joint tuberculosis. Eur Spine J. 22:Suppl 4. 556–566. 2013.
Article23. Saavedra-Lozano J, Mejías A, Ahmad N, Peromingo E, Ardura MI, Guillen S, et al. Changing trends in acute osteomyelitis in children: impact of methicillin-resistant staphylococcus aureus infections. J Pediatr Orthop. 28:569–575. 2008.24. Smith AS, Weinstein MA, Mizushima A, Coughlin B, Hayden SP, Lakin MM, et al. MR imaging characteristics of tuberculous spondylitis vs vertebral osteomyelitis. AJR Am J Roentgenol. 153:399–405. 1989.
Article25. Tins BJ, Cassar-Pullicino VN. MR imaging of spinal infection. Semin Musculoskelet Radiol. 8:215–229. 2004.
Article26. Yoon YK, Jo YM, Kwon HH, Yoon HJ, Lee EJ, Park SY, et al. Differential diagnosis between tuberculous spondylodiscitis and pyogenic spontaneous spondylodiscitis: a multicenter descriptive and comparative study. Spine J. 15:1764–1771. 2015.
Article27. Zilkens KW, Peters KM, Schwanitz BM. New inflammation markers for early detection of spondylodiscitis. Eur Spine J. 1:152–155. 1992.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Pyogenic and Tuberculous Spondylitis
- Preoperative Gadolinium-enhanced Magnetic Resonance Images on Infectious Spondylitis
- Comparison of Pyogenic Spondylitis and Tuberculous Spondylitis
- Streptococcus Spondylitis Concomitant Infectious Endocarditis: A Case Report
- Diagnosis and Treatment of Tuberclous Spondylitis and Pyogenic Spondylitis in Atypical Cases