Cancer Res Treat.
2015 Oct;47(4):813-822. 10.4143/crt.2014.238.
Prognostic Significance of Defining L-Cell Type on the Biologic Behavior of Rectal Neuroendocrine Tumors in Relation with Pathological Parameters
- Affiliations
-
- 1Department of Pathology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Department of Pathology, Wonju Severance Christian Hospital, Yonsei University Wonju College of Medicine, Wonju, Korea. meeyon@yonsei.ac.kr
- 3Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 4Department of Pathology, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 5Department of Pathology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- 6Department of Pathology, Department of Pathology, Inha University Hospital, Inha University School of Medicine, Incheon, Korea.
- 7Department of Pathology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 8Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 9Department of Pathology, Chonnam National University Hospital, Chonnam National University Medical School, Gwangju, Korea.
- 10Department of Pathology, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea.
- 11Department of Pathology, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea.
- 12Department of Pathology, Inje University Ilsan Paik Hospital, Inje Univeristy College of Medicine, Goyang, Korea.
- 13Department of Pathology, Dong-A University Hospital, Dong-A University College of Medicine, Busan, Korea.
- 14Department of Pathology, Chonbuk National University Hospital, Chonbuk National University Medical School, Jeonju, Korea.
- 15Department of Pathology, Inje University Busan Paik Hospital,Inje University College of Medicine, Busan, Korea.
- 16Department of Pathology, Soon Chun Hyang University Hospital, Soon Chun Hyang University College of Medicine, Seoul, Korea.
- 17Department of Pathology, Inje University Seoul Paik Hospital, Inje University College of Medicine, Seoul, Korea.
- 18Department of Pathology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 19Department of Pathology, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea.
- 20Division of Statistics in Institute of ifestyle Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
- KMID: 2403401
- DOI: http://doi.org/10.4143/crt.2014.238
Abstract
- PURPOSE
In 2010, the World Health Organization categorized L-cell type neuroendocrine tumors (NETs) as tumors of uncertain malignancy, while all others were classified as malignant. However, the diagnostic necessity of L-cell immunophenotyping is unclear, as are tumor stage and grade that may guide diagnosis and management. To clarify the predictive markers of rectal neuroendocrine neoplasms (NENs), 5- and 10-year overall survival (OS) was analyzed by pathological parameters including L-cell phenotype.
MATERIALS AND METHODS
A total of 2,385 rectal NENs were analyzed from our previous multicenter study and a subset of 170 rectal NENs was immunophenotyped.
RESULTS
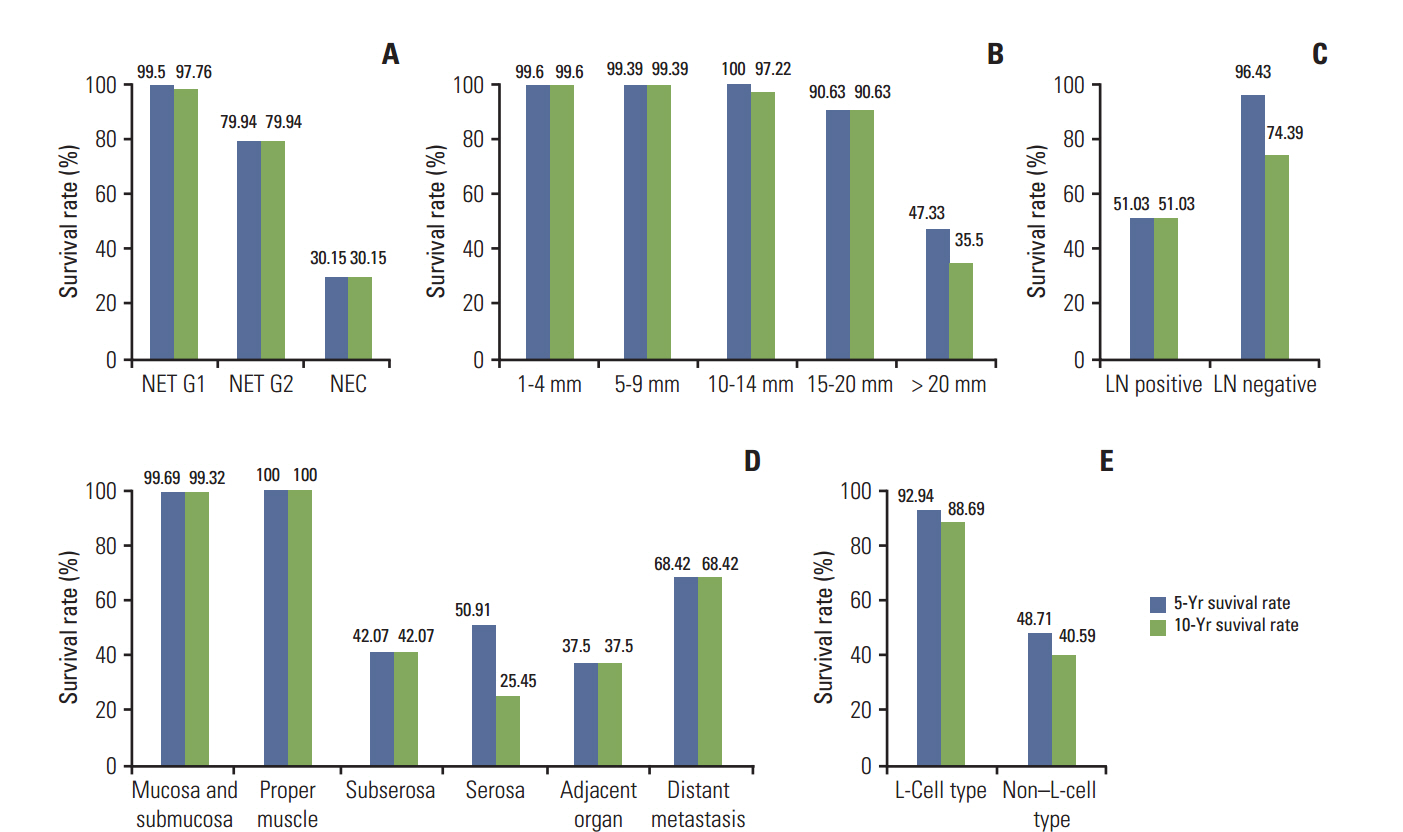
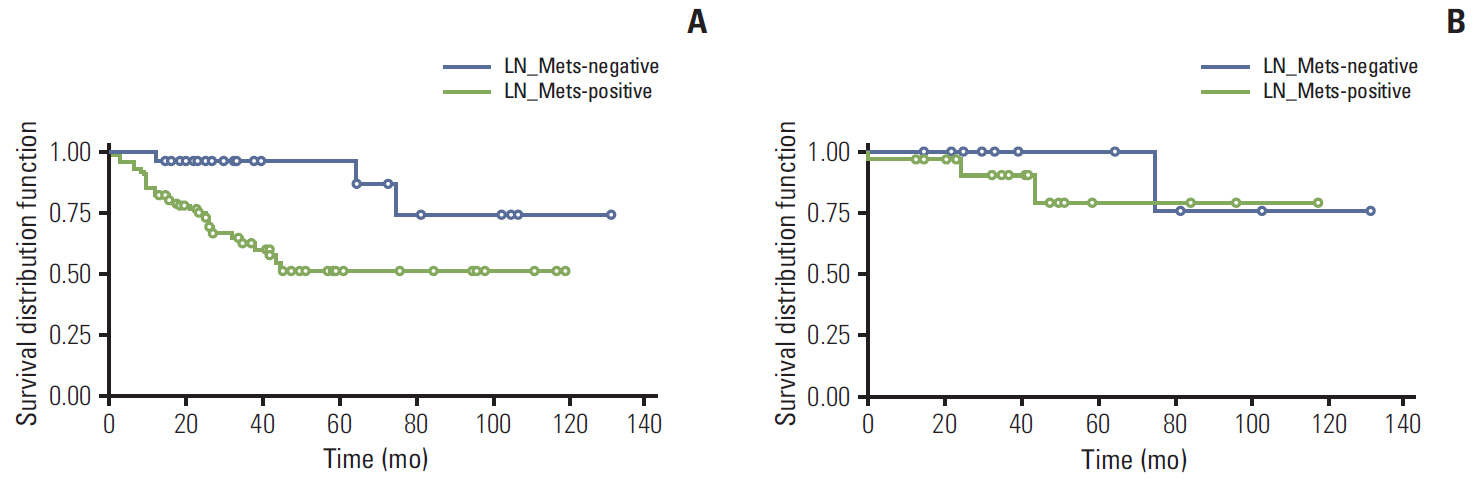
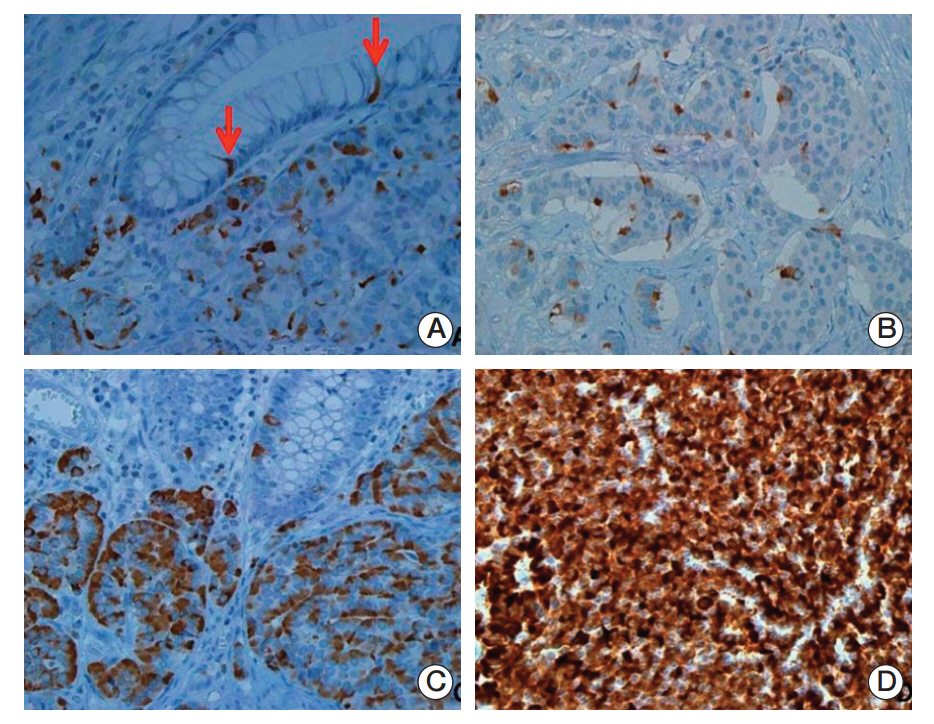
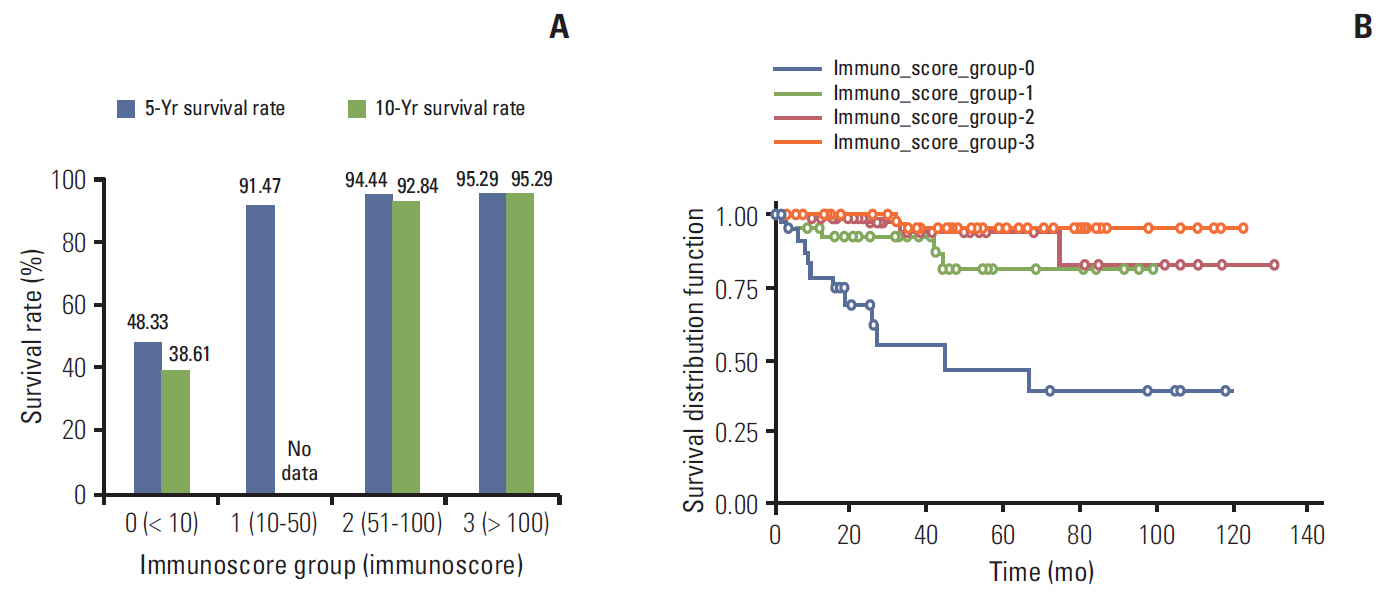
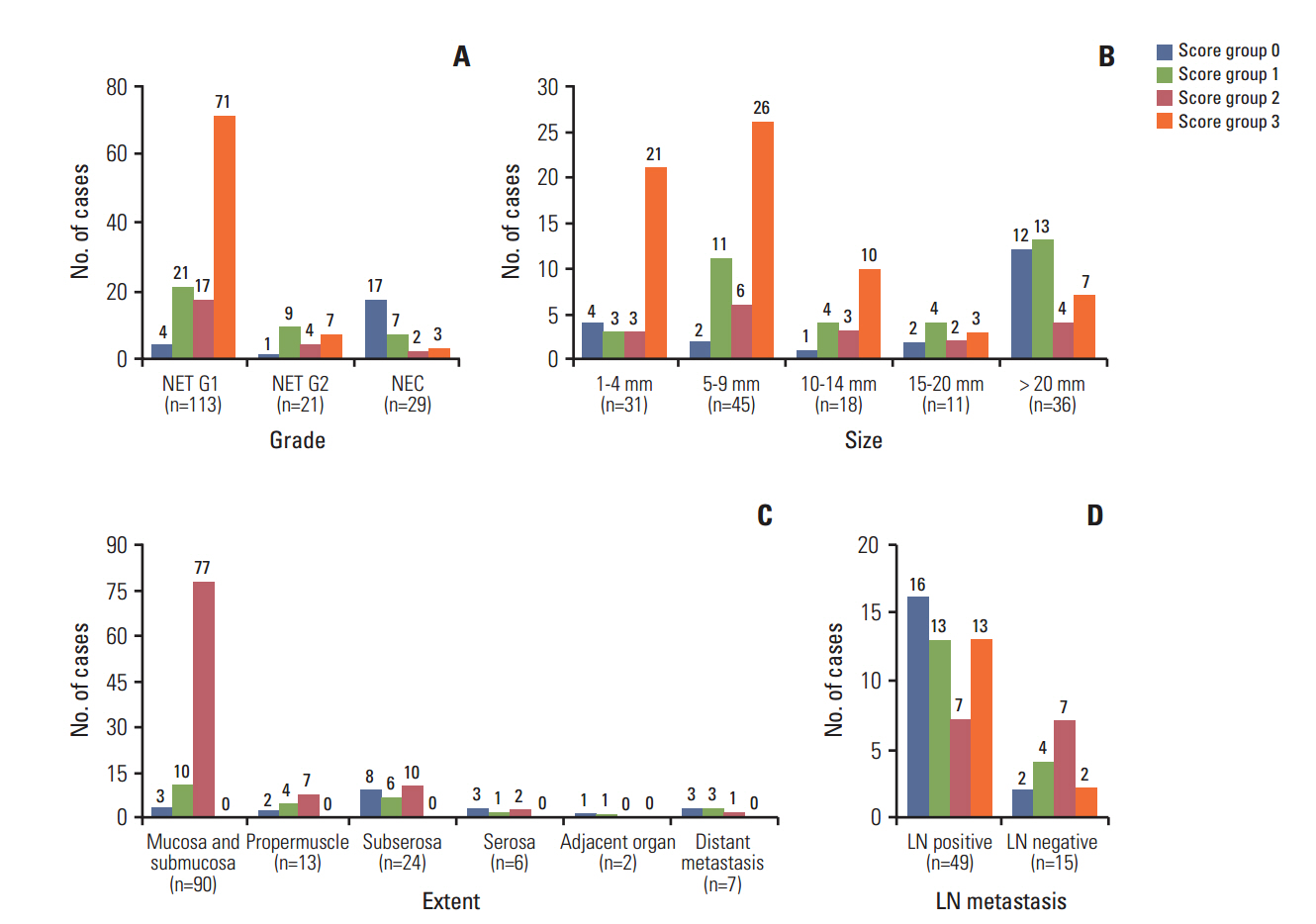
In univariate survival analysis, tumor grade (p < 0.0001), extent (p < 0.0001), size (p < 0.0001), lymph node metastasis (p=0.0063), and L-cell phenotype (p < 0.0001) showed significant correlation with the prognosis of rectal NENs; however, none of these markers achieved independent significance in multivariate analysis. The 10-year OS of tumors of NET grade 1, < 10 mm, the mucosa/submucosa was 97.58%, 99.47%, and 99.03%, respectively. L-Cell marker, glucagon II (GLP-1&2), with a cut off score of > 10, is useful in defining L-Cell type. In this study, an L-cell immunophenotype was found in 83.5% of all rectal NENs and most, but not all L-cell type tumors were NET G1, small (< 10 mm) and confined to the mucosa/submucosa.
CONCLUSION
From these results, the biological behavior of rectal NENs does not appear to be determined by L-cell type alone but instead by a combination of pathological parameters.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008; 26:3063–72.
Article2. Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003; 97:934–59.
Article3. Cho MY, Kim JM, Sohn JH, Kim MJ, Kim KM, Kim WH, et al. Current Trends of the incidence and pathological diagnosis of gastroenteropancreatic neuroendocrine tumors (GEPNETs) in Korea 2000-2009: multicenter study. Cancer Res Treat. 2012; 44:157–65.
Article4. Lawrence B, Gustafsson BI, Chan A, Svejda B, Kidd M, Modlin IM. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011; 40:1–18.
Article5. Tsikitis VL, Wertheim BC, Guerrero MA. Trends of incidence and survival of gastrointestinal neuroendocrine tumors in the United States: a SEER analysis. J Cancer. 2012; 3:292–302.
Article6. Frilling A, Akerstrom G, Falconi M, Pavel M, Ramos J, Kidd M, et al. Neuroendocrine tumor disease: an evolving landscape. Endocr Relat Cancer. 2012; 19:R163–85.
Article7. Ito T, Sasano H, Tanaka M, Osamura RY, Sasaki I, Kimura W, et al. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol. 2010; 45:234–43.
Article8. Maggard MA, O'Connell JB, Ko CY. Updated population-based review of carcinoid tumors. Ann Surg. 2004; 240:117–22.
Article9. Rindi G, Bordi C, La Rosa S, Solcia E, Delle Fave G; Gruppo Italiano Patologi Apparato Digerente (GIPAD), et al. Gastroenteropancreatic (neuro)endocrine neoplasms: the histology report. Dig Liver Dis. 2011; 43 Suppl 4:S356–60.
Article10. Hauso O, Gustafsson BI, Kidd M, Waldum HL, Drozdov I, Chan AK, et al. Neuroendocrine tumor epidemiology: contrasting Norway and North America. Cancer. 2008; 113:2655–64.11. Caplin M, Sundin A, Nillson O, Baum RP, Klose KJ, Kelestimur F, et al. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: colorectal neuroendocrine neoplasms. Neuroendocrinology. 2012; 95:88–97.
Article12. Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. 4th ed. Lyon: IARC Press;2010.13. Kulke MH, Benson AB 3rd, Bergsland E, Berlin JD, Blaszkowsky LS, Choti MA, et al. Neuroendocrine tumors. J Natl Compr Canc Netw. 2012; 10:724–64.
Article14. Lee SH, Kim BC, Chang HJ, Sohn DK, Han KS, Hong CW, et al. Rectal neuroendocrine and L-cell tumors: diagnostic dilemma and therapeutic strategy. Am J Surg Pathol. 2013; 37:1044–52.15. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17:1471–4.
Article16. Jernman J, Valimaki MJ, Louhimo J, Haglund C, Arola J. The novel WHO 2010 classification for gastrointestinal neuroendocrine tumours correlates well with the metastatic potential of rectal neuroendocrine tumours. Neuroendocrinology. 2012; 95:317–24.
Article17. Colonoscopy Study Group of Korean Society of Coloproctology. Clinical characteristics of colorectal carcinoid tumors. J Korean Soc Coloproctol. 2011; 27:17–20.18. Yamagishi D, Matsubara N, Noda M, Yamano T, Tsukamoto K, Kuno T, et al. Clinicopathological characteristics of rectal carcinoid patients undergoing surgical resection. Oncol Lett. 2012; 4:910–4.
Article19. Hirata I, Akutagawa H, Nishikawa T, Yasumato S, Sanomuras M, Egashira Y. Clinicopathological study of colorectal carcinoid tumor, focusing on the indication for endoscopic mucosal resection (EMR). Stomach Intest. 2005; 40:182–93.20. Tabata H, Matsumoto T, Kuwano Y, Yao T, Iida M. Rectal carcinoid tumor 10 mm in diameter accompanied by multiple liver metastases, report of a case. Stomach Intest. 2005; 40:215–20.21. Cho MY, Kang YK, Kim KM, Chang HK, Chang HJ, Chang MS, et al. Porposal for creating a guideline for cancer registration of the gastrointestinal tumors (I). Korean J Pathol. 2008; 42:140–50.22. Jung ES, Kang YK, Cho MY, Kim JM, Lee WA, Lee HE, et al. Update on the proposal for creating a guideline for cancer registration of the gastrointestinal tumors (I-2). Korean J Pathol. 2012; 46:443–53.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuroendocrine Tumors of the Female Reproductive Tract: A Literature Review
- Endoscopic Treatment Outcome of Rectal Neuroendocrine Tumors Removed by Ligation-assisted Endoscopic Submucosal Resection
- Pituitary Neuroendocrine Tumor: Is It Benign or Malignant?
- Neuroendocrine Tumors of the Larynx: Four Cases
- Prognostic Value of Pathological Parameters in Renal Cell Carcinoma