J Vet Sci.
2018 Jan;19(1):13-20. 10.4142/jvs.2018.19.1.13.
Comparison of the characteristics of canine adipose tissue-derived mesenchymal stem cells extracted from different sites and at different passage numbers
- Affiliations
-
- 1Laboratory of Immunology, Department of Microbiological Science, Faculty of Veterinary, Universidad de la República, Montevideo 11600, Uruguay. jacmaiso@gmail.com
- 2Laboratory of Embryology and Cellular Differentiation, Hospital de ClÃnicas de Porto Alegre, Porto Alegre, RS 90035-903, Brazil.
- 3Biostatistics, Hospital de ClÃnicas de Porto Alegre, Porto Alegre, RS 90035-903, Brazil.
- 4Laboratory for Vaccine Research, Department of Biotechnology, Instituto de Higiene, Faculty of Medicine, Universidad de la República, Montevideo 11600, Uruguay.
- 5Laboratory of Genetics, Faculty of Veterinary, Universidad de la República, Montevideo 11600, Uruguay.
- KMID: 2402690
- DOI: http://doi.org/10.4142/jvs.2018.19.1.13
Abstract
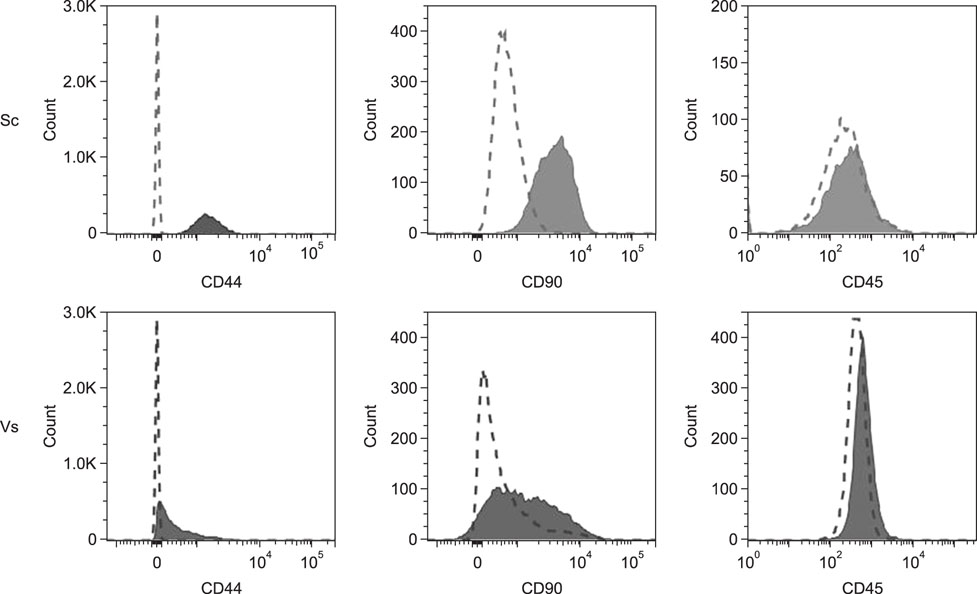
- Mesenchymal stem cells (MSCs) have desirable characteristics for use in therapy in animal models and veterinary medicine, due to their capacity of inducing tissue regeneration and immunomodulation. The objective of this study was to evaluate the differences between canine adipose tissue-derived MSCs (AD-MSCs) extracted from subcutaneous (Sc) and visceral (Vs) sites. Surface antigenic markers, in vitro differentiation, and mineralized matrix quantification of AD-MSCs at different passages (P₄, P₆, and P₈) were studied. Immunophenotypic analysis showed that AD-MSCs from both sites were CD44+, CD90+, and CD45−. Moreover, they were able, in vitro, to differentiate into fat, cartilage, and bone. Sc-AD-MSCs preserve in vitro multipotentiality up to P₈, but Vs-AD-MSCs only tri-differentiated up to P₄. In addition, compared to Vs-AD-MSCs, Sc-AD-MSCs had greater capacity for in vitro mineralized matrix synthesis. In conclusion, Sc-AD-MSCs have advantages over Vs-AD-MSCs, as Sc AD-MSCs preserve multipotentiality during a greater number of passages, have more osteogenic potential, and require less invasive extraction.
MeSH Terms
Figure
Reference
-
1. An SY, Han J, Lim HJ, Park SY, Kim JH, Do BR, Kim JH. Valproic acid promotes differentiation of hepatocyte-like cells from whole human umbilical cord-derived mesenchymal stem cells. Tissue Cell. 2014; 46:127–135.
Article2. Anderson P, Souza-Moreira L, Morell M, Caro M, O'Valle F, Gonzalez-Rey E, Delgado M. Adipose-derived mesenchymal stromal cells induce immunomodulatory macrophages which protect from experimental colitis and sepsis. Gut. 2013; 62:1131–1141.
Article3. Baglioni S, Cantini G, Poli G, Francalanci M, Squecco R, Di Franco A, Borgogni E, Frontera S, Nesi G, Liotta F, Lucchese M, Perigli G, Francini F, Forti G, Serio M, Luconi M. Functional differences in visceral and subcutaneous fat pads originate from differences in the adipose stem cell. PLoS One. 2012; 7:e36569.
Article4. Baglioni S, Francalanci M, Squecco R, Lombardi A, Cantini G, Angeli R, Gelmini S, Guasti D, Benvenuti S, Annunziato F, Bani D, Liotta F, Francini F, Perigli G, Serio M, Luconi M. Characterization of human adult stem-cell populations isolated from visceral and subcutaneous adipose tissue. FASEB J. 2009; 23:3494–3505.
Article5. Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissuederived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013; 15:641–648.
Article6. Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991; 9:641–650.
Article7. Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, Lu M, Gautam SC, Chopp M. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003; 73:778–786.
Article8. Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001; 189:54–63.
Article9. Guercio A, Di Bella S, Casella S, Di Marco P, Russo C, Piccione G. Canine mesenchymal stem cells (MSCs): characterization in relation to donor age and adipose tissue-harvesting site. Cell Biol Int. 2013; 37:789–798.
Article10. Hocking SL, Chisholm DJ, James DE. Studies of regional adipose transplantation reveal a unique and beneficial interaction between subcutaneous adipose tissue and the intra-abdominal compartment. Diabetologia. 2008; 51:900–902.
Article11. Kang BJ, Ryu HH, Park SS, Koyama Y, Kikuchi M, Woo HM, Kim WH, Kweon OK. Comparing the osteogenic potential of canine mesenchymal stem cells derived from adipose tissues, bone marrow, umbilical cord blood, and Wharton's jelly for treating bone defects. J Vet Sci. 2012; 13:299–310.
Article12. Katz AJ, Tholpady A, Tholpady SS, Shang H, Ogle RC. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005; 23:412–423.
Article13. Krasnodembskaya A, Song Y, Fang X, Gupta N, Serikov V, Lee JW, Matthay MA. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 2010; 28:2229–2238.
Article14. Laschke MW, Harder Y, Amon M, Martin I, Farhadi J, Ring A, Torio-Padron N, Schramm R, Rücker M, Junker D, Häufel JM, Carvalho C, Heberer M, Germann G, Vollmar B, Menger MD. Angiogenesis in tissue engineering: breathing life into constructed tissue substitutes. Tissue Eng. 2006; 12:2093–2104.
Article15. Lee KS, Kang HW, Lee HT, Kim HJ, Kim CL, Song JY, Lee KW, Cha SH. Sequential sub-passage decreases the differentiation potential of canine adipose-derived mesenchymal stem cells. Res Vet Sci. 2014; 96:267–275.
Article16. Martinello T, Bronzini I, Maccatrozzo L, Mollo A, Sampaolesi M, Mascarello F, Decaminada M, Patruno M. Canine adipose-derived-mesenchymal stem cells do not lose stem features after a long-term cryopreservation. Res Vet Sci. 2011; 91:18–24.
Article17. Mitchell A, Rivas KA, Smith R 3rd, Watts AE. Cryopreservation of equine mesenchymal stem cells in 95% autologous serum and 5% DMSO does not alter post-thaw growth or morphology in vitro compared to fetal bovine serum or allogeneic serum at 20 or 95% and DMSO at 10 or 5%. Stem Cell Res Ther. 2015; 6:231.
Article18. Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Di Halvorsen Y, Storms RW, Goh B, Kilroy G, Wu X, Gimble JM. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006; 24:376–385.
Article19. Niemeyer P, Szalay K, Luginbühl R, Südkamp NP, Kasten P. Transplantation of human mesenchymal stem cells in a non-autogenous setting for bone regeneration in a rabbit critical-size defect model. Acta Biomater. 2010; 6:900–908.
Article20. Nijnik A, Hancock RE. The roles of cathelicidin LL-37 in immune defences and novel clinical applications. Curr Opin Hematol. 2009; 16:41–47.
Article21. Peptan IA, Hong L, Mao JJ. Comparison of osteogenic potentials of visceral and subcutaneous adipose-derived cells of rabbits. Plast Reconstr Surg. 2006; 117:1462–1470.
Article22. Requicha JF, Viegas CA, Albuquerque CM, Azevedo JM, Reis RL, Gomes ME. Effect of anatomical origin and cell passage number on the stemness and osteogenic differentiation potential of canine adipose-derived stem cells. Stem Cell Rev. 2012; 8:1211–1222.
Article23. Riekstina U, Cakstina I, Parfejevs V, Hoogduijn M, Jankovskis G, Muiznieks I, Muceniece R, Ancans J. Embryonic stem cell marker expression pattern in human mesenchymal stem cells derived from bone marrow, adipose tissue, heart and dermis. Stem Cell Rev. 2009; 5:378–386.
Article24. Screven R, Kenyon E, Myers MJ, Yancy HF, Skasko M, Boxer L, Bigley EC 3rd, Borjesson DL, Zhu M. Immunophenotype and gene expression profile of mesenchymal stem cells derived from canine adipose tissue and bone marrow. Vet Immunol Immunopathol. 2014; 161:21–31.
Article25. Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, Fraser JK, Hedrick MH. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005; 54:132–141.
Article26. Szaraz P, Librach M, Maghen L, Iqbal F, Barretto TA, Kenigsberg S, Gauthier-Fisher A, Librach CL. In vitro differentiation of first trimester human umbilical cord perivascular cells into contracting cardiomyocyte-like cells. Stem Cells Int. 2016; 2016:7513252.27. Takemitsu H, Zhao D, Yamamoto I, Harada Y, Michishita M, Arai T. Comparison of bone marrow and adipose tissue-derived canine mesenchymal stem cells. BMC Vet Res. 2012; 8:150.
Article28. Terraciano P, Garcez T, Ayres L, Durli I, Baggio M, Kuhl CP, Laurino C, Passos E, Paz AH, Cirne-Lima E. Cell therapy for chemically induced ovarian failure in mice. Stem Cells Int. 2014; 2014:720753.
Article29. Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008; 7:410–420.
Article30. Vieira NM, Brandalise V, Zucconi E, Secco M, Strauss BE, Zatz M. Isolation, characterization, and differentiation potential of canine adipose-derived stem cells. Cell Transplant. 2010; 19:279–289.
Article31. Volk SW, Theoret C. Translating stem cell therapies: the role of companion animals in regenerative medicine. Wound Repair Regen. 2013; 21:382–394.
Article32. Weiss ML, Troyer DL. Stem cells in the umbilical cord. Stem Cell Rev. 2006; 2:155–162.
Article33. Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007; 25:2648–2659.
Article34. Yaneselli K, Filomeno A, Semiglia G, Arce C, Rial A, Muñoz N, Moreno M, Erickson K, Maisonnave J. Allogeneic stem cell transplantation for bone regeneration of a nonunion defect in a canine. Vet Med Res Rep. 2013; 4:39–44.35. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001; 7:211–228.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chondrogenesis of Mesenchymal Stem Cell Derived from Canine Adipose Tissue
- Effect of donor age on the proliferation and multipotency of canine adipose-derived mesenchymal stem cells
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Adipose Tissue - Adequate, Accessible Regenerative Material
- Stem cell properties of cells derived from canine periodontal ligament