J Korean Med Sci.
2017 Oct;32(10):1595-1602. 10.3346/jkms.2017.32.10.1595.
Poorly Differentiated Clusters in Colorectal Adenocarcinomas Share Biological Similarities with Micropapillary Patterns as well as Tumor Buds
- Affiliations
-
- 1Department of Pathology, Hallym University Kangnam Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea. jwkim@hallym.or.kr
- 2Department of Surgery, Hallym University Kangnam Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea.
- KMID: 2400430
- DOI: http://doi.org/10.3346/jkms.2017.32.10.1595
Abstract
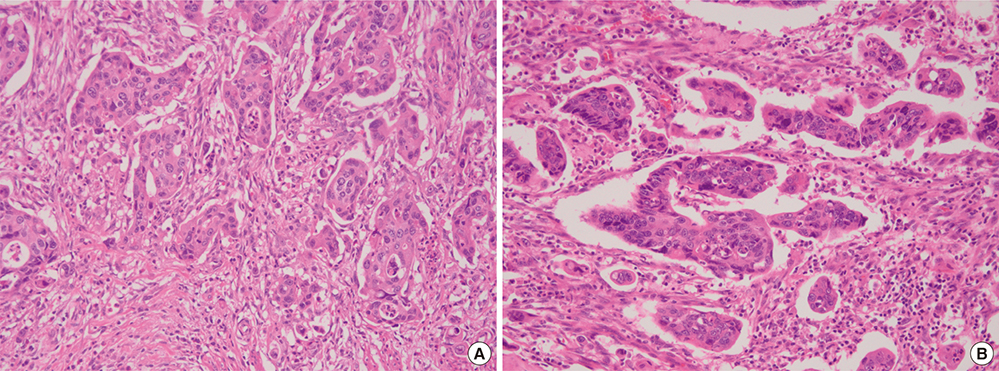
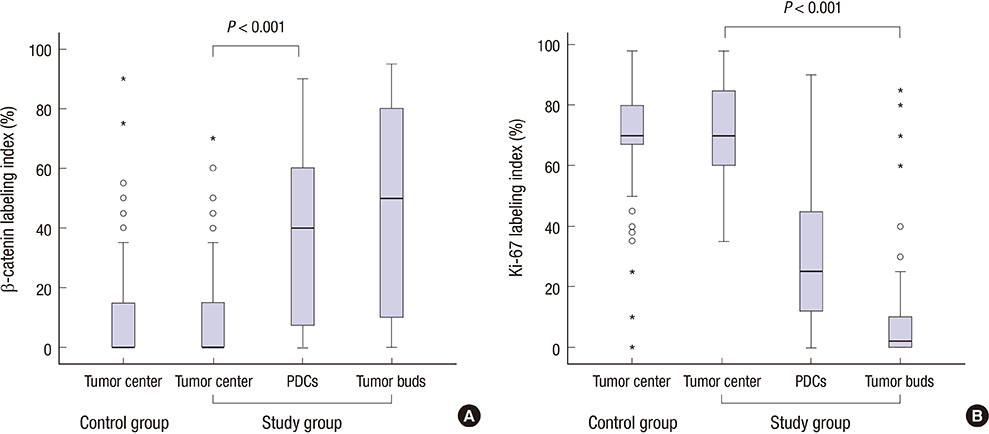
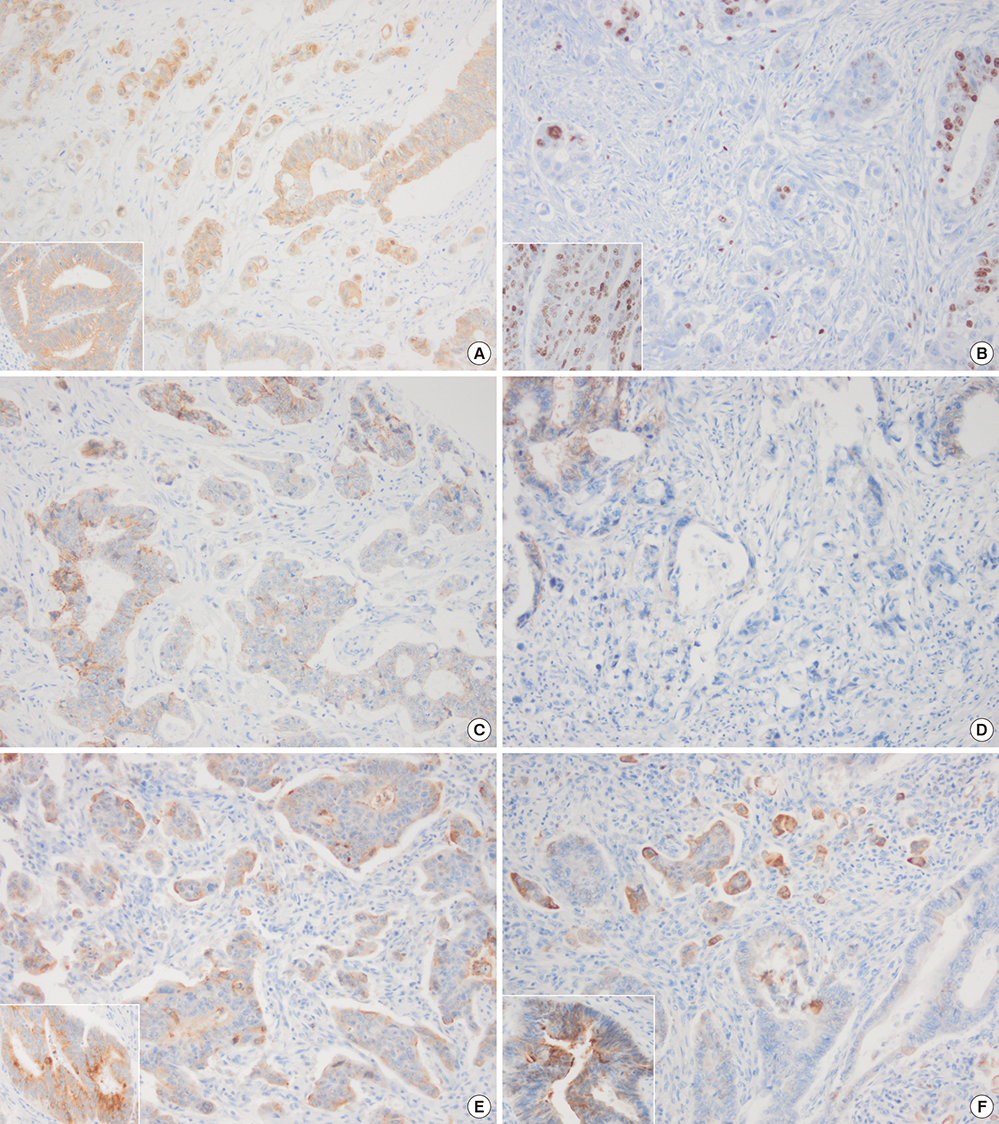
- In colorectal carcinoma, poorly differentiated clusters (PDCs) are a poor prognostic indicator and show morphological continuity and behavioral similarities to micropapillary patterns (MPPs) as well as tumor buds (TBs). Epithelial-mesenchymal transition (EMT) and inhibition of cancer-stromal interactions may contribute to the development of PDCs. To clarify the biological nature of PDCs, we examined immunohistochemical stainings for β-catenin, Ki-67, E-cadherin, epithelial cell adhesion molecule (EpCAM), MUC1, and epithelial membrane antigen (EMA), which are associated with EMT and cancer-stromal interactions. The expression frequencies and patterns of PDCs, TBs, and differentiated neoplastic glands from the tumor center (TC) were compared. In the study group (117 cases), the nuclear β-catenin staining index was higher in PDCs (37.3%) and TBs (43.3%) than in neoplastic glands from TC (8.9%, P < 0.001). The mean Ki-67 labeling index in TC was 71.5%, whereas it was decreased in PDCs (31.2%) and TBs (10.2%, P < 0.001). E-cadherin and EpCAM displayed a tendency to be found along the cell membrane in TC samples (91.5% and 92.3%, respectively), whereas they showed loss of membranous staining in PDC (44.4% and 36.8%, respectively) and TB samples (60.7% and 68.4%, respectively). An inside-out pattern for MUC1 and EMA was frequently observed in PDC (48.7% and 45.3%, respectively) and TB samples (46.2% and 45.3%, respectively), but not in TC samples. Our data demonstrate that there is a pathogenetic overlap among PDCs, TBs, and MPPs and suggest that they might represent sequential growth patterns that branch from common biological processes such as dedifferentiation and alteration in cancer-stromal interactions.
MeSH Terms
Figure
Reference
-
1. Kim JW, Shin MK, Kim BC. Clinicopathologic impacts of poorly differentiated cluster-based grading system in colorectal carcinoma. J Korean Med Sci. 2015; 30:16–23.2. Graham RP, Vierkant RA, Tillmans LS, Wang AH, Laird PW, Weisenberger DJ, Lynch CF, French AJ, Slager SL, Raissian Y, et al. Tumor budding in colorectal carcinoma: confirmation of prognostic significance and histologic cutoff in a population-based cohort. Am J Surg Pathol. 2015; 39:1340–1346.3. Hamilton SR, Bosman FT, Boffetta P, Ilyas M, Morreau H, Nakamura S, Quirke P, Riboli E, Sobin LH. Carcinoma of the colon and rectum. In : Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon: International Agenecy for Research on Cancer (IARC) Press;2010. p. 134–146.4. Zlobec I, Molinari F, Martin V, Mazzucchelli L, Saletti P, Trezzi R, De Dosso S, Vlajnic T, Frattini M, Lugli A. Tumor budding predicts response to anti-EGFR therapies in metastatic colorectal cancer patients. World J Gastroenterol. 2010; 16:4823–4831.5. Zlobec I, Lugli A. Epithelial mesenchymal transition and tumor budding in aggressive colorectal cancer: tumor budding as oncotarget. Oncotarget. 2010; 1:651–661.6. Ueno H, Price AB, Wilkinson KH, Jass JR, Mochizuki H, Talbot IC. A new prognostic staging system for rectal cancer. Ann Surg. 2004; 240:832–839.7. Ueno H, Mochizuki H, Hashiguchi Y, Ishiguro M, Kajiwara Y, Sato T, Shimazaki H, Hase K, Talbot IC. Histological grading of colorectal cancer: a simple and objective method. Ann Surg. 2008; 247:811–818.8. Reggiani Bonetti L, Barresi V, Bettelli S, Domati F, Palmiere C. Poorly differentiated clusters (PDC) in colorectal cancer: what is and ought to be known. Diagn Pathol. 2016; 11:31.9. Dawson H, Lugli A. Molecular and pathogenetic aspects of tumor budding in colorectal cancer. Front Med (Lausanne). 2015; 2:11.10. Ueno H, Hase K, Hashiguchi Y, Shimazaki H, Tanaka M, Miyake O, Masaki T, Shimada Y, Kinugasa Y, Mori Y, et al. Site-specific tumor grading system in colorectal cancer: multicenter pathologic review of the value of quantifying poorly differentiated clusters. Am J Surg Pathol. 2014; 38:197–204.11. Nassar H, Pansare V, Zhang H, Che M, Sakr W, Ali-Fehmi R, Grignon D, Sarkar F, Cheng J, Adsay V. Pathogenesis of invasive micropapillary carcinoma: role of MUC1 glycoprotein. Mod Pathol. 2004; 17:1045–1050.12. Lillehoj EP, Lu W, Kiser T, Goldblum SE, Kim KC. MUC1 inhibits cell proliferation by a beta-catenin-dependent mechanism. Biochim Biophys Acta. 2007; 1773:1028–1038.13. Lee HJ, Eom DW, Kang GH, Han SH, Cheon GJ, Oh HS, Han KH, Ahn HJ, Jang HJ, Han MS. Colorectal micropapillary carcinomas are associated with poor prognosis and enriched in markers of stem cells. Mod Pathol. 2013; 26:1123–1131.14. Barresi V, Branca G, Vitarelli E, Tuccari G. Micropapillary pattern and poorly differentiated clusters represent the same biological phenomenon in colorectal cancer: a proposal for a change in terminology. Am J Clin Pathol. 2014; 142:375–383.15. Colon and rectum. In : Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer-Verlag;2010. p. 143–164.16. Ueno H, Kajiwara Y, Shimazaki H, Shinto E, Hashiguchi Y, Nakanishi K, Maekawa K, Katsurada Y, Nakamura T, Mochizuki H, et al. New criteria for histologic grading of colorectal cancer. Am J Surg Pathol. 2012; 36:193–201.17. Dawson H, Koelzer VH, Karamitopoulou E, Economou M, Hammer C, Muller DE, Lugli A, Zlobec I. The apoptotic and proliferation rate of tumour budding cells in colorectal cancer outlines a heterogeneous population of cells with various impacts on clinical outcome. Histopathology. 2014; 64:577–584.18. Jung A, Schrauder M, Oswald U, Knoll C, Sellberg P, Palmqvist R, Niedobitek G, Brabletz T, Kirchner T. The invasion front of human colorectal adenocarcinomas shows co-localization of nuclear beta-catenin, cyclin D1, and p16INK4A and is a region of low proliferation. Am J Pathol. 2001; 159:1613–1617.19. Bruun J, Kolberg M, Nesland JM, Svindland A, Nesbakken A, Lothe RA. Prognostic significance of β-catenin, E-cadherin, and SOX9 in colorectal cancer: results from a large population-representative series. Front Oncol. 2014; 4:118.20. Cserni G. Reversed polarity of the glandular epithelial cells in micropapillary carcinoma of the large intestine and the EMA/MUC1 immunostain. Pathology. 2014; 46:527–532.21. Pai K, Baliga P, Shrestha BL. E-cadherin expression: a diagnostic utility for differentiating breast carcinomas with ductal and lobular morphologies. J Clin Diagn Res. 2013; 7:840–844.22. Gosens MJ, van Kempen LC, van de Velde CJ, van Krieken JH, Nagtegaal ID. Loss of membranous Ep-CAM in budding colorectal carcinoma cells. Mod Pathol. 2007; 20:221–232.23. Koelzer VH, Zlobec I, Lugli A. Tumor budding in colorectal cancer--ready for diagnostic practice? Hum Pathol. 2016; 47:4–19.24. Barresi V, Reggiani Bonetti L, Branca G, Di Gregorio C, Ponz de Leon M, Tuccari G. Colorectal carcinoma grading by quantifying poorly differentiated cell clusters is more reproducible and provides more robust prognostic information than conventional grading. Virchows Arch. 2012; 461:621–628.25. Kaihara T, Kusaka T, Nishi M, Kawamata H, Imura J, Kitajima K, Itoh-Minami R, Aoyama N, Kasuga M, Oda Y, et al. Dedifferentiation and decreased expression of adhesion molecules, E-cadherin and ZO-1, in colorectal cancer are closely related to liver metastasis. J Exp Clin Cancer Res. 2003; 22:117–123.26. Zlobec I, Lugli A, Baker K, Roth S, Minoo P, Hayashi S, Terracciano L, Jass JR. Role of APAF-1, E-cadherin and peritumoral lymphocytic infiltration in tumour budding in colorectal cancer. J Pathol. 2007; 212:260–268.27. van der Gun BT, Melchers LJ, Ruiters MH, de Leij LF, McLaughlin PM, Rots MG. EpCAM in carcinogenesis: the good, the bad or the ugly. Carcinogenesis. 2010; 31:1913–1921.28. Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, Knuechel R, Kirchner T. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci USA. 2001; 98:10356–10361.29. Sangoi AR, Beck AH, Amin MB, Cheng L, Epstein JI, Hansel DE, Iczkowski KA, Lopez-Beltran A, Oliva E, Paner GP, et al. Interobserver reproducibility in the diagnosis of invasive micropapillary carcinoma of the urinary tract among urologic pathologists. Am J Surg Pathol. 2010; 34:1367–1376.30. Acs G, Khakpour N, Kiluk J, Lee MC, Laronga C. The presence of extensive retraction clefts in invasive breast carcinomas correlates with lymphatic invasion and nodal metastasis and predicts poor outcome: a prospective validation study of 2742 consecutive cases. Am J Surg Pathol. 2015; 39:325–337.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pulmonary Adenocarcinoma with a Micropapillary Pattern Detected by Bronchial Washing: A Brief Case Report
- Expression of Rb , p16 and Cyclin D1 Proteins in Gastric Adenoma and Adenocarcinoma
- An immunohistochemical study of the expression of p53 protein in colon cancer
- Micropapillary Carcinoma of the Gallbladder
- The Significance of the Expression of p53, E-cadherin, nm23, CD44, and Tumor Angiogenesis in Colorectal Adenocarcinoma