Asia Pac Allergy.
2013 Jan;3(1):50-58. 10.5415/apallergy.2013.3.1.50.
Murine subcutaneous immunotherapy models with beneficial immunological and physiological effects
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul National University Medical Research Center, Seoul 110-744, Korea. shcho@plaza.snu.ac.kr
- 2Institute of Allergy and Clinical Immunology, Seoul National University Medical Research Center, Seoul 110-744, Korea.
- 3Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam 463-802, Korea.
- KMID: 2397335
- DOI: http://doi.org/10.5415/apallergy.2013.3.1.50
Abstract
- BACKGROUND
Immunotherapy was introduced 100 years ago and has a unique role in the treatment of allergic diseases in that only immunotherapy can induce long-term immunological tolerance. However, only a few mouse models of immunotherapy have been developed so far.
OBJECTIVE
We tried to establish murine immunotherapy models that have similar findings in human using subcutaneous rush immunotherapy-like schedule.
METHODS
To determine the maximal safe or maximal tolerable dose, injection dose was doubled twice a day from the dose of sensitization. Mice with established asthma using ovalbumin (OVA) were repeatedly injected with OVA from the dose of sensitization subcutaneously twice a day: after reaching to the maximal safe or maximal tolerable dose, mice were injected with each dose either 10 times or 24 times.
RESULTS
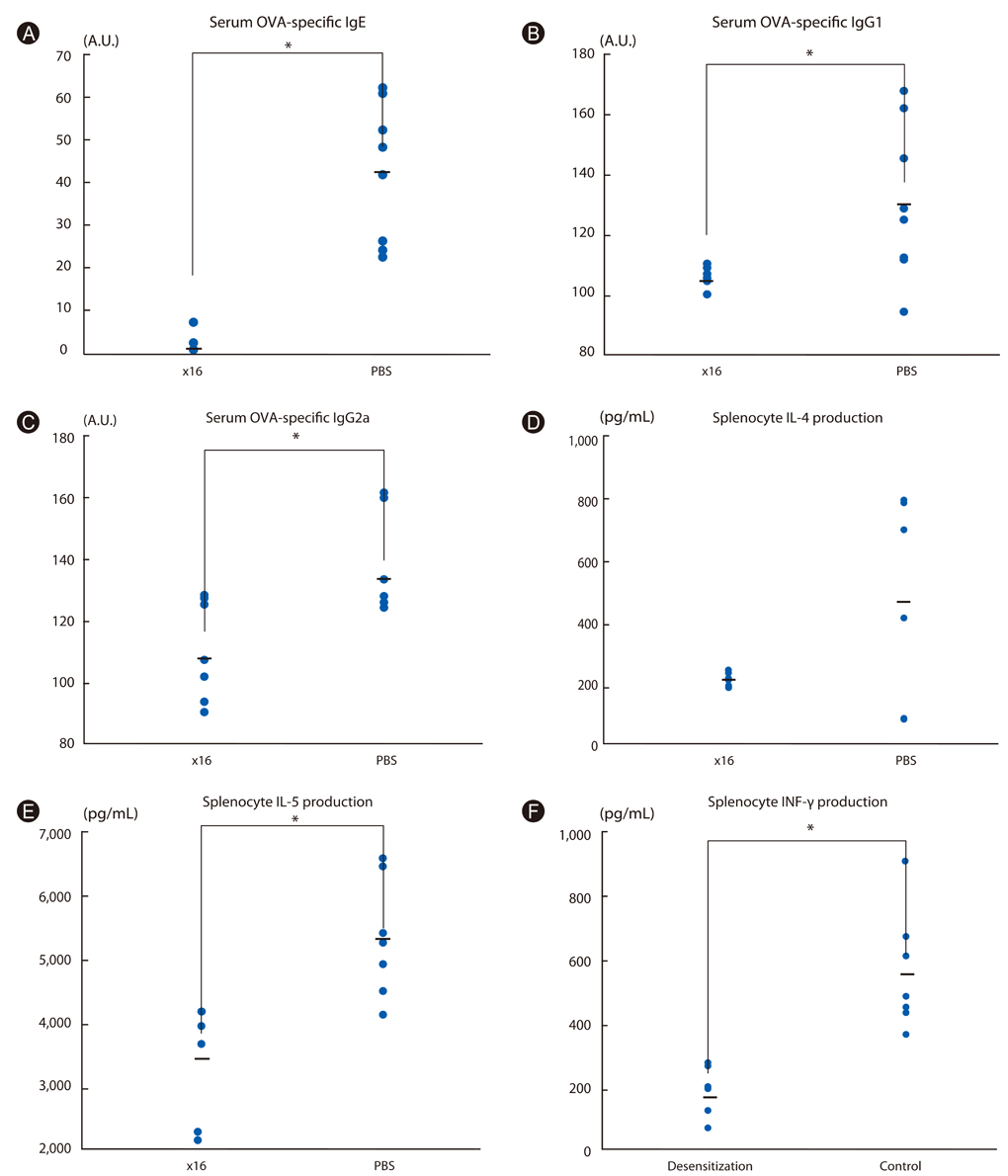
Short term immunotherapy (10 times) with the maximal safe and tolerable dose of OVA showed decreased IL-5 production, decreased IL-5/INF-γ ratio, and increased IgG2a/IgG1 but there was no significant difference in airway hyperresponsiveness (AHR) or airway inflammation. Prolonged immunotherapy (24 times) with the maximal tolerable dose not only decreased cytokine productions of IL-5 and even INF-γ, but also decreased IgE, IgG1 and even IgG2a production. Remarkably, the prolonged immunotherapy provided a protective effect on AHR.
CONCLUSION
This study suggested immunotherapy models with some beneficial immunological and physiological effects in murine asthma.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Influence of the Adjuvants and Genetic Background on the Asthma Model Using Recombinant Der f 2 in Mice
Yoon-Seok Chang, Yoon-Keun Kim, Seong Gyu Jeon, Sae-Hoon Kim, Sun-Sin Kim, Heung-Woo Park, Kyung-Up Min, You-Young Kim, Sang-Heon Cho
Immune Netw. 2013;13(6):295-300. doi: 10.4110/in.2013.13.6.295.Great learning, much networking, and friendship
Yoon-Seok Chang
Asia Pac Allergy. 2015;5(4):191-192. doi: 10.5415/apallergy.2015.5.4.191.
Reference
-
1. Korean Academy of Asthma, Allergy and Clinical Immunology, Center for Chronic Obstructive Airway Diseases. Korean asthma management guideline for adult 2011. Available from: www.allergy.or.kr.2. Noon L. Prophylactic inoculation against hay fever. Lancet. 1911; 1:1572–1573.
Article3. Akkoc T, Akdis M, Akdis CA. Update in the mechanisms of allergen-specific immunotheraphy. Allergy Asthma Immunol Res. 2011; 3:11–20.
Article4. Han DH, Rhee CS. Sublingual immunotherapy in allergic rhinitis. Asia Pac Allergy. 2011; 1:123–129.
Article5. Fujita H, Meyer N, Akdis M, Akdis CA. Mechanisms of immune tolerance to allergens. Chem Immunol Allergy. 2012; 96:30–38.
Article6. Jacobsen L, Wahn U, Bilo MB. Allergen-specific immunotherapy provides immediate, long-term and preventive clinical effects in children and adults: the effects of immunotherapy can be categorised by level of benefit -the centenary of allergen specific subcutaneous immunotherapy. Clin Transl Allergy. 2012; 2:8.
Article7. Eifan AO, Shamji MH, Durham SR. Long-term clinical and immunological effects of allergen immunotherapy. Curr Opin Allergy Clin Immunol. 2011; 11:586–593.
Article8. Chang YS, Kim YK, Bahn JW, Kim SH, Park HW, Kim TB, Cho SH, Min KU, Kim YY. Comparison of asthma phenotypes using different sensitizing protocols in mice. Korean J Intern Med. 2005; 20:152–158.
Article9. Park Y, Chang YS, Lee SW, Cho SY, Kim YK, Min KU, Kim YY, Cho SH, Sung YC. The enhanced effect of a hexameric deoxyriboguanosine run conjugation to CpG oligodeoxynucleotides on protection against allergic asthma. J Allergy Clin Immunol. 2001; 108:570–576.
Article10. Chung Y, Choi J, Chang YS, Cho SH, Kang CY. Preventive and therapeutic effects of oral tolerance in a murine model of asthma. Immunobiology. 2002; 206:408–423.
Article11. Chang YS, Kim YK, Min KU, Kim YY, Seong YC, Cho SH. Conjugation of 3' hexameric deoxyriboguanosine run to phosphodiester CpG oligodeoxynucleotides can inhibit allergen-specific IgE synthesis with less risk of splenomegaly. J Allergy Clin Immunol. 2005; 116:1388–1390.
Article12. Chang YS, Kim YK, Kwon HS, Park HW, Min KU, Kim YY, Cho SH. The effect of CpG-oligodeoxynucleotides with different backbone structures and 3' hexameric deoxyriboguanosine run conjugation on the treatment of asthma in mice. J Korean Med Sci. 2009; 24:860–866.
Article13. Johansen P, Senti G, Maria Martínez Gómez J, Kündig TM. Medication with antihistamines impairs allergen-specific immunotherapy in mice. Clin Exp Allergy. 2008; 38:512–519.
Article14. Fitzhugh DJ, Lockey RF. Allergen immunotherapy: a history of the first 100 years. Curr Opin Allergy Clin Immunol. 2011; 11:554–559.15. Calderón M, Cardona V, Demoly P. One hundred years of allergen immunotherapy European Academy of Allergy and Clinical Immunology celebration: review of unanswered questions. Allergy. 2012; 67:462–476.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Radiation-induced abscopal effect and its enhancement by programmed cell death 1 blockade in the hepatocellular carcinoma: A murine model study
- Effect of BCG Immunotherapy on the Cytokine Production and Antitumor Activity against MBT - 2 Mouse Bladder Tumor
- New Lung Transplantation Technique for Immunological Study: Subcutaneous Transplantation of Lung Tissue
- Physiological spaces and multicompartmental pharmacokinetic models
- Sublingual immunotherapeutics