Asia Pac Allergy.
2013 Oct;3(4):257-265. 10.5415/apallergy.2013.3.4.257.
Nuts 'n' guts: transport of food allergens across the intestinal epithelium
- Affiliations
-
- 1Centre for Cellular and Molecular Biology, School of Life and Environmental Sciences, Faculty of Science, Engineering and Built Environment, Deakin University, Burwood, VIC 3125, Australia.
- 2NeuroAllergy Research Laboratory, School of Life and Environmental Sciences, Faculty of Science, Engineering and Built Environment, Deakin University, Geelong, VIC 3216, Australia. cenk@deakin.edu.au
- KMID: 2397303
- DOI: http://doi.org/10.5415/apallergy.2013.3.4.257
Abstract
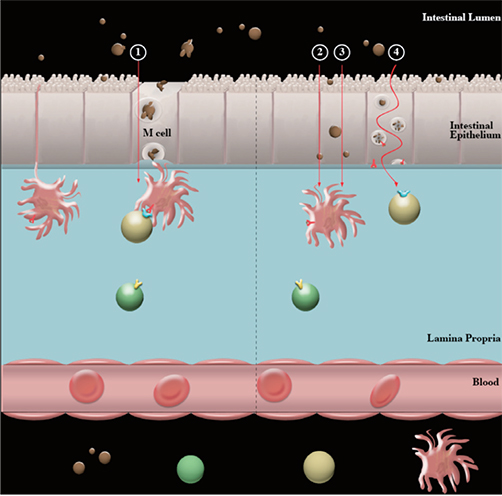
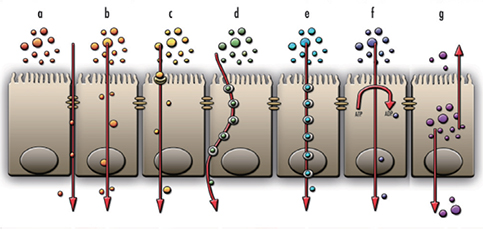
- The increase in the incidence of food allergy is a growing problem for the western world. This review will focus on the findings from several macromolecular epithelial transport experiments and drug permeability studies to provide a recent comprehension of food allergen intestinal epithelial cell transport and the allergen-epithelial relationship. Specifically, this review will aim to answer whether allergens can permeate the intestinal barrier directly via intestinal epithelial cells, and whether this mode of transport affects downstream immune reactions. By improving our understanding of the interactions which take place during exposure of food allergens with the intestinal epithelium, we can begin to understand whether the epithelial barrier plays a major role in the allergic sensitization process rather than simply restricting the entry of allergens to the underlying lamina propria.
Keyword
MeSH Terms
Figure
Reference
-
1. Bock SA, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001-2006. J Allergy Clin Immunol. 2007; 119:1016–1018.
Article2. Hourihane JO'B, Kilburn SA, Nordlee JA, Hefle SL, Taylor SL, Warner JO. An evaluation of the sensitivity of subjects with peanut allergy to very low doses of peanut protein: a randomized, double-blind, placebo-controlled food challenge study. J Allergy Clin Immunol. 1997; 100:596–600.
Article3. Mullins RJ, Dear KB, Tang ML. Characteristics of childhood peanut allergy in the Australian Capital Territory, 1995 to 2007. J Allergy Clin Immunol. 2009; 123:689–693.
Article4. Venter C, Hasan Arshad S, Grundy J, Pereira B, Bernie Clayton C, Voigt K, Higgins B, Dean T. Time trends in the prevalence of peanut allergy: three cohorts of children from the same geographical location in the UK. Allergy. 2010; 65:103–108.
Article5. Kotz D, Simpson CR, Sheikh A. Incidence, prevalence, and trends of general practitioner-recorded diagnosis of peanut allergy in England, 2001 to 2005. J Allergy Clin Immunol. 2011; 127:623–630.e1.
Article6. Grundy J, Matthews S, Bateman B, Dean T, Arshad SH. Rising prevalence of allergy to peanut in children: data from 2 sequential cohorts. J Allergy Clin Immunol. 2002; 110:784–789.
Article7. Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: a 5-year follow-up study. J Allergy Clin Immunol. 2003; 112:1203–1207.8. Hofmann AM, Scurlock AM, Jones SM, Palmer KP, Lokhnygina Y, Steele PH, Kamilaris J, Burks AW. Safety of a peanut oral immunotherapy protocol in children with peanut allergy. J Allergy Clin Immunol. 2009; 124:286–291.e6.
Article9. Kopper RA, Odum NJ, Sen M, Helm RM, Stanley JS, Burks AW. Peanut protein allergens: the effect of roasting on solubility and allergenicity. Int Arch Allergy Immunol. 2005; 136:16–22.
Article10. Astwood JD, Leach JN, Fuchs RL. Stability of food allergens to digestion in vitro. Nat Biotechnol. 1996; 14:1269–1273.
Article11. Burks AW, Shin D, Cockrell G, Stanley JS, Helm RM, Bannon GA. Mapping and mutational analysis of the IgE-binding epitopes on Ara h 1, a legume vicilin protein and a major allergen in peanut hypersensitivity. Eur J Biochem. 1997; 245:334–339.
Article12. Stanley JS, King N, Burks AW, Huang SK, Sampson H, Cockrell G, Helm RM, West CM, Bannon GA. Identification and mutational analysis of the immunodominant IgE binding epitopes of the major peanut allergen Ara h 2. Arch Biochem Biophys. 1997; 342:244–253.13. Rabjohn P, Helm EM, Stanley JS, West CM, Sampson HA, Burks AW, Bannon GA. Molecular cloning and epitope analysis of the peanut allergen Ara h 3. J Clin Invest. 1999; 103:535–542.
Article14. Maleki SJ, Kopper RA, Shin DS, Park CW, Compadre CM, Sampson H, Burks AW, Bannon GA. Structure of the major peanut allergen Ara h 1 may protect IgE-binding epitopes from degradation. J Immunol. 2000; 164:5844–5849.
Article15. Maleki SJ, Viquez O, Jacks T, Dodo H, Champagne ET, Chung SY, Landry SJ. The major peanut allergen, Ara h 2, functions as a trypsin inhibitor, and roasting enhances this function. J Allergy Clin Immunol. 2003; 112:190–195.
Article16. Starkl P, Krishnamurthy D, Szalai K, Felix F, Lukschal A, Oberthuer D, Sampson HA, Swoboda I, Betzel C, Untersmayr E, Jensen-Jarolim E. Heating affects structure, enterocyte adsorption and signalling, as well as immunogenicity of the peanut allergen ara h 2. Open Allergy J. 2011; 4:24–34.
Article17. Chu DK, Llop-Guevara A, Walker TD, Flader K, Goncharova S, Boudreau JE, Moore CL, Seunghyun In T, Waserman S, Coyle AJ, Kolbeck R, Humbles AA, Jordana M. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J Allergy Clin Immunol. 2013; 131:187–200.e8.
Article18. Kraehenbuhl JP, Neutra MR. Epithelial M cells: differentiation and function. Annu Rev Cell Dev Biol. 2000; 16:301–332.
Article19. Dreskin SC, Tripputi MT, Aubrey MT, Mustafa SS, Atkins D, Leo HL, Song B, Schlichting D, Talwar H, Wang Q, Freed BM. Peanut-allergic subjects and their peanut-tolerant siblings have large differences in peanut-specific IgG that are independent of HLA class II. Clin Immunol. 2010; 137:366–373.
Article20. Vadas P, Wai Y, Burks W, Perelman B. Detection of peanut allergens in breast milk of lactating women. JAMA. 2001; 285:1746–1748.
Article21. So AL, Small G, Sperber K, Becker K, Oei E, Tyorkin M, Mayer L. Factors affecting antigen uptake by human intestinal epithelial cell lines. Dig Dis Sci. 2000; 45:1130–1137.22. Roth-Walter F, Berin MC, Arnaboldi P, Escalante CR, Dahan S, Rauch J, Jensen-Jarolim E, Mayer L. Pasteurization of milk proteins promotes allergic sensitization by enhancing uptake through Peyer's patches. Allergy. 2008; 63:882–890.
Article23. Moreno FJ, Rubio LA, Olano A, Clemente A. Uptake of 2S albumin allergens, Ber e 1 and Ses i 1, across human intestinal epithelial Caco-2 cell monolayers. J Agric Food Chem. 2006; 54:8631–8639.
Article24. Mine Y, Zhang JW. Surfactants enhance the tight-junction permeability of food allergens in human intestinal epithelial Caco-2 cells. Int Arch Allergy Immunol. 2003; 130:135–142.
Article25. Tukker JJ. Characterization of transport over epithelial barriers. In : Lehr CM, editor. Cell culture models of biological barriers: in vitro test systems for drug absorption and delivery. London: Taylor & Francis;2002. p. 52–61.26. MacDonald TT, Weinel A, Spencer J. HLA-DR expression in human fetal intestinal epithelium. Gut. 1988; 29:1342–1348.
Article27. Lopes LM, Hughson E, Anstee Q, O'Neil D, Katz DR, Chain BM. Vectorial function of major histocompatibility complex class II in a human intestinal cell line. Immunology. 1999; 98:16–26.
Article28. Hatano R, Yamada K, Iwamoto T, Maeda N, Emoto T, Shimizu M, Totsuka M. Antigen presentation by small intestinal epithelial cells uniquely enhances IFN-γ secretion from CD4+ intestinal intraepithelial lymphocytes. Biochem Biophys Res Commun. 2013; 435:592–596.
Article29. Lin XP, Almqvist N, Telemo E. Human small intestinal epithelial cells constitutively express the key elements for antigen processing and the production of exosomes. Blood Cells Mol Dis. 2005; 35:122–128.
Article30. van Niel G, Raposo G, Candalh C, Boussac M, Hershberg R, Cerf-Bensussan N, Heyman M. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology. 2001; 121:337–349.
Article31. Mallegol J, Van Niel G, Lebreton C, Lepelletier Y, Candalh C, Dugave C, Heath JK, Raposo G, Cerf-Bensussan N, Heyman M. T84-intestinal epithelial exosomes bear MHC class II/peptide complexes potentiating antigen presentation by dendritic cells. Gastroenterology. 2007; 132:1866–1876.
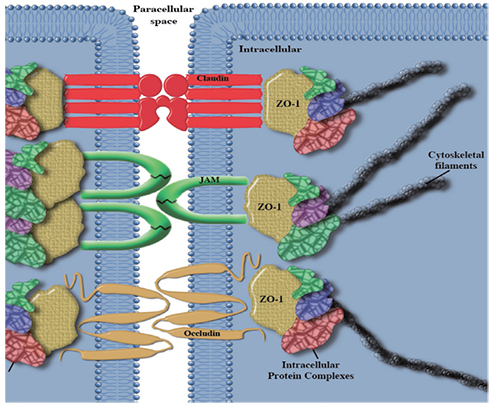
Article32. Feldman GJ, Mullin JM, Ryan MP. Occludin: structure, function and regulation. Adv Drug Deliv Rev. 2005; 57:883–917.
Article33. Severson EA, Parkos CA. Structure and function of JAM proteins. In : Ley K, editor. Adhesion molecules: function and inhibition. Basel: Birkhauser;2007. p. 271–288.34. Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE. Structure and function of claudins. Biochim Biophys Acta. 2008; 1778:631–645.
Article35. Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998; 273:29745–29753.
Article36. Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, Matsui T, Tsukita S, Furuse M, Tsukita S. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006; 126:741–754.
Article37. Itoh M, Morita K, Tsukita S. Characterization of ZO-2 as a MAGUK family member associated with tight as well as adherens junctions with a binding affinity to occludin and alpha catenin. J Biol Chem. 1999; 274:5981–5986.38. Haskins J, Gu L, Wittchen ES, Hibbard J, Stevenson BR. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J Cell Biol. 1998; 141:199–208.
Article39. Niessen CM. Tight junctions/adherens junctions: basic structure and function. J Invest Dermatol. 2007; 127:2525–2532.
Article40. Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog Biophys Mol Biol. 2003; 81:1–44.41. Assimakopoulos SF, Scopa CD, Charonis A, Spiliopoulou I, Georgiou C, Nikolopoulou V, Vagianos CE. Experimental obstructive jaundice disrupts intestinal mucosal barrier by altering occludin expression: beneficial effect of bombesin and neurotensin. J Am Coll Surg. 2004; 198:748–757.42. Rosenberg GA, Yang Y. Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia. Neurosurg Focus. 2007; 22:E4.
Article43. Schmitz H, Barmeyer C, Fromm M, Runkel N, Foss HD, Bentzel CJ, Riecken EO, Schulzke JD. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 1999; 116:301–309.
Article44. Kominsky SL, Argani P, Korz D, Evron E, Raman V, Garrett E, Rein A, Sauter G, Kallioniemi OP, Sukumar S. Loss of the tight junction protein claudin-7 correlates with histological grade in both ductal carcinoma in situ and invasive ductal carcinoma of the breast. Oncogene. 2003; 22:2021–2033.
Article45. Sawada N, Murata M, Kikuchi K, Osanai M, Tobioka H, Kojima T, Chiba H. Tight junctions and human diseases. Med Electron Microsc. 2003; 36:147–156.
Article46. Chambers SJ, Wickham MS, Regoli M, Bertelli E, Gunning PA, Nicoletti C. Rapid in vivo transport of proteins from digested allergen across pre-sensitized gut. Biochem Biophys Res Commun. 2004; 325:1258–1263.
Article47. Wan H, Winton HL, Soeller C, Tovey ER, Gruenert DC, Thompson PJ, Stewart GA, Taylor GW, Garrod DR, Cannell MB, Robinson C. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest. 1999; 104:123–133.
Article48. Bodinier M, Legoux MA, Pineau F, Triballeau S, Segain JP, Brossard C, Denery-Papini S. Intestinal translocation capabilities of wheat allergens using the Caco-2 cell line. J Agric Food Chem. 2007; 55:4576–4583.
Article49. Moreno FJ, Clemente A. 2S albumin storage proteins: what makes them food allergens? Open Biochem J. 2008; 2:16–28.
Article50. Song CH, Liu ZQ, Huang S, Zheng PY, Yang PC. Probiotics promote endocytic allergen degradation in gut epithelial cells. Biochem Biophys Res Commun. 2012; 426:135–140.
Article51. Matsubara T, Akiyama Y, Oshima K, Okajima T, Nadano D, Matsuda T. Dephosphorylation reduces passage of ovalbumin antigen through intestinal epithelial Caco-2 cell monolayers. J Biochem. 2013; 153:347–354.
Article52. Sewekow E, Bimczok D, Kahne T, Faber-Zuschratter H, Kessler LC, Seidel-Morgenstern A, Rothkotter HJ. The major soyabean allergen P34 resists proteolysis in vitro and is transported through intestinal epithelial cells by a caveolae-mediated mechanism. Br J Nutr. 2012; 108:1603–1611.53. van Boxtel EL, van den Broek LA, Koppelman SJ, Gruppen H. Legumin allergens from peanuts and soybeans: effects of denaturation and aggregation on allergenicity. Mol Nutr Food Res. 2008; 52:674–682.
Article54. Blanc F, Vissers YM, Adel-Patient K, Rigby NM, Mackie AR, Gunning AP, Wellner NK, Skov PS, Przybylski-Nicaise L, Ballmer-Weber B, Zuidmeer-Jongejan L, Szepfalusi Z, Ruinemans-Koerts J, Jansen AP, Bernard H, Wal JM, Savelkoul HF, Wichers HJ, Mills EN. Boiling peanut Ara h 1 results in the formation of aggregates with reduced allergenicity. Mol Nutr Food Res. 2011; 55:1887–1894.
Article55. Kroghsbo S, Rigby N, Vissers Y, Mills C, Madsen C. Roasting or heating increases elicitation capacity of peanut allergens but does not affect their sensitisation potential in a brown Norway rat model for food allergy. Clin Transl Allergy. 2011; 1:O20.
Article