Past, present, and future of pharmacovigilance in Korea
- Affiliations
-
- 1Drug Safety Monitoring Center, Seoul National University Hospital, Seoul 03080, Korea. shcho@snu.ac.kr
- 2Seoul National University Hospital Regional Pharmacovigilance Center, Seoul 03080, Korea.
- 3Department of Internal Medicine, Seoul National University College of Medicine, Seoul 03080, Korea.
- KMID: 2396942
- DOI: http://doi.org/10.5415/apallergy.2017.7.3.173
Abstract
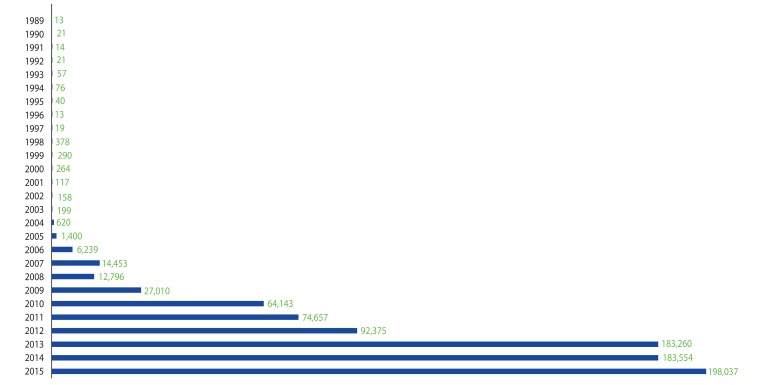
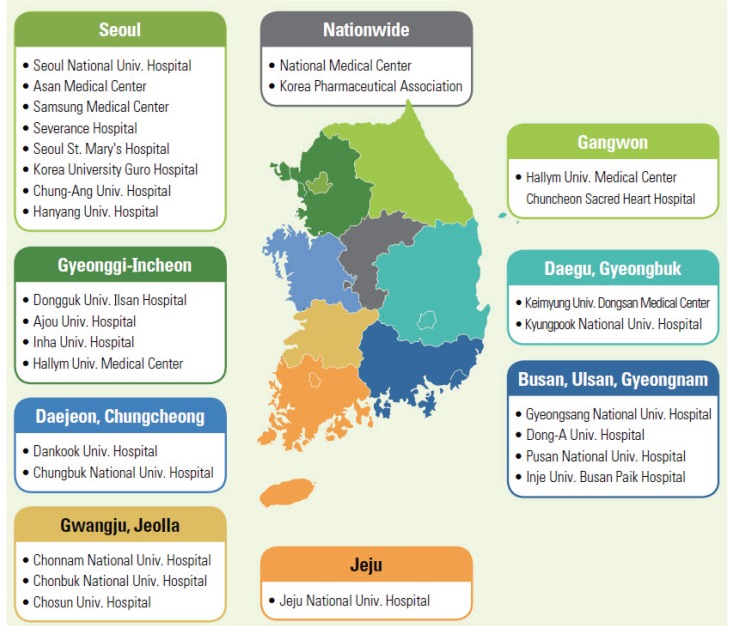
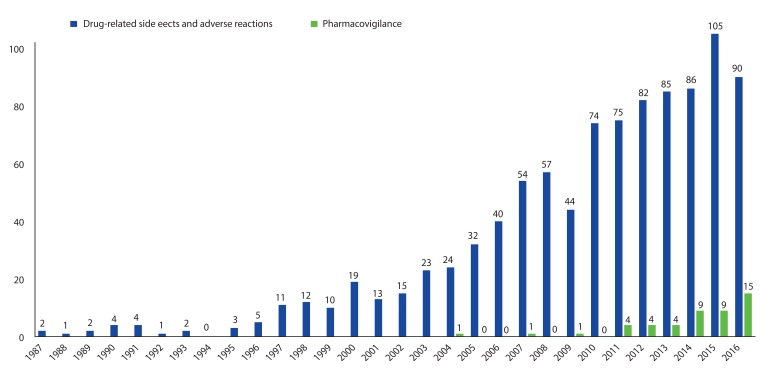
- As an essential part of patient safety, pharmacovigilance is of worldwide interest and should expand its scope and focus on new emerging issues. South Korea has been making continuous efforts in the field of pharmacovigilance for the last 3 decades since voluntary adverse drug reaction (ADR) reporting system was first launched in 1988. Korea joined the World Health Organization Program for International Drug Monitoring in 1992, and the activities of Pharmacovigilance Research Network, Korean Society for Pharmacoepidemiology and Risk Management, and Regional Pharmacovigilance Center (RPVC) have contributed to the remarkable progress in the pharmacovigilance area and global status. RPVCs have played pivotal roles in establishment of pharmacovigilance system in Korea by monitoring voluntary ADR reports. RPVCs started with 3 hospitals in 2006 and have now expanded to 27 hospitals nationwide. The Korea Institute of Drug Safety & Risk Management was established in 2012 and in charge of operating the decentralized national pharmacovigilance system. The voluntary report of ADR, which is the basis of current pharmacovigilance system, has various limitations and an active surveillance system can be the overarching alternative. This change in pharmacovigilance paradigm is a global trend and Korea has excellent infrastructure such as broad distribution of electronic medical recording systems and a nationwide single healthcare insurance. As a result, the pharmacovigilance in Korea is now expected to progress to a new active surveillance system from traditional spontaneous reporting system.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
Severe Cutaneous Adverse Reactions to Antiepileptic Drugs: A Nationwide Registry-Based Study in Korea
Chan Sun Park, Dong Yoon Kang, Min Gyu Kang, Sujeong Kim, Young Min Ye, Sae Hoon Kim, Hye-Kyung Park, Jung-Won Park, Young Hee Nam, Min-Suk Yang, Young-Koo Jee, Jae Woo Jung, Sang Hyon Kim, Cheol-Woo Kim, Mi-Yeong Kim, Joo Hee Kim, Jaechun Lee, Jun-Gyu Lee, Sang Hyun Kim, Hyen O La, Min-Hye Kim, Seoung Ju Park, Young-Il Koh, Sang-Min Lee, Yong Eun Kwon, Hyun Jung Jin, Hee-Kyoo Kim, Hye-Ryun Kang, Jeong-Hee Choi,
Allergy Asthma Immunol Res. 2019;11(5):709-722. doi: 10.4168/aair.2019.11.5.709.Screening of severe adverse drug reaction by electronic medical record search
Cheol-Woo Kim
Allergy Asthma Respir Dis. 2018;6(3):135-136. doi: 10.4168/aard.2018.6.3.135.Incidence and Economic Burden of Adverse Drug Reactions in Hospitalization: A Prospective Study in Korea
Bomi Seo, Min-Suk Yang, So-Young Park, Bo Young Park, Jung-Hyun Kim, Woo-Jung Song, Hyouk-Soo Kwon, Yoon-Seok Chang, You Sook Cho, Sae-Hoon Kim, Tae-Bum Kim
J Korean Med Sci. 2023;38(8):e56. doi: 10.3346/jkms.2023.38.e56.
Reference
-
1. The importance of pharmacovigilance. Safety monitoring of medicinal products. Geneva: World Health Organization;2002.2. Royall BW. International aspects of drug monitoring. Role of the World Health Organization. WHO Chron. 1971; 25:445–451. PMID: 5131672.3. International drug monitoring: the role of national centres. Report of a WHO meeting. World Health Organ Tech Rep Ser. 1972; 498:1–25. PMID: 4625548.4. Venulet J, Helling-Borda M. WHO’s international drug monitoring--the formative years, 1968-1975: preparatory, pilot and early operational phases. Drug Saf. 2010; 33:e1–e23. PMID: 20553053.5. Members of the WHO Programme for International Drug Monitoring [Internet]. Uppsala (Sweden): Uppsala Monitoring Centre;2017. cited 2017 July 13. Available from: https://www.who-umc.org/global-pharmacovigilance/members/who-programme-members.6. Choi NK, Park BJ. Adverse drug reaction surveillance system in Korea. J Prev Med Public Health. 2007; 40:278–284. PMID: 17693730.
Article7. Choi NK, Lee J, Park BJ. Recent international initiatives of drug safety management. J Korean Med Assoc. 2012; 55:819–826.
Article8. Shin JY, Jung SY, Ahn SH, Lee SH, Kim SJ, Seong JM, Chung SY, Park BJ. New initiatives for pharmacovigilance in South Korea: introducing the Korea Institute of Drug Safety and Risk Management (KIDS). Pharmacoepidemiol Drug Saf. 2014; 23:1115–1122. PMID: 25251052.
Article9. Shin YS, Lee YW, Choi YH, Park B, Jee YK, Choi SK, Kim EG, Park JW, Hong CS. Spontaneous reporting of adverse drug events by Korean regional pharmacovigilance centers. Pharmacoepidemiol Drug Saf. 2009; 18:910–915. PMID: 19621345.
Article10. Lee JH. About pharmacovigilance corporation. J Pharmacoepidemiol Risk Manag. 2009; 2:1–2.11. History of RPVC [Internet]. Anyang (Korea): Korea Institute of Drug Safety & Risk Management;2017. cited 2017 June 14. Available from: https://www.drugsafe.or.kr/iwt/ds/en/community/EgovHistoryPvCenter.do.12. Regional PV Center [Internet]. Anyang (Korea): Korea Institute of Drug Safety & Risk Management;2017. cited 2017 June 14. Available from: https://www.drugsafe.or.kr/iwt/ds/en/community/EgovCenterGuide.do;jsessionid=114mSnG2895xyBUactCB3nJ50CwGAwX170BY7ENcnxrnDJsQ2aq9zR1HdxCOrP0q.webint_2_servlet_engine1.13. Relief of Injury from ADR [Internet]. Anyang (Korea): Korea Institute of Drug Safety & Risk Management;2017. cited 2017 June 14. Available from: https://www.drugsafe.or.kr/iwt/ds/en/introduction/EgovRelevantStatutoryProvisions.do;jsessionid=VAXlIicn1X0XjhmvZFhVm4o8Z3Uy5YEOOlZI0nAtfyt4hD6HgMiqzwpLoCkHKaDz.webint_2_servlet_engine1.14. Seong JM, Park BJ. Recent advance in pharmacovigilance activities of World Health Organization and U.S. Food and Drug Administration. Korean Public Health Res. 2015; 41:19–28.15. Platt R, Carnahan RM, Brown JS, Chrischilles E, Curtis LH, Hennessy S, Nelson JC, Racoosin JA, Robb M, Schneeweiss S, Toh S, Weiner MG. The U.S. Food and Drug Administration's Mini-Sentinel program: status and direction. Pharmacoepidemiol Drug Saf. 2012; 21(Suppl 1):1–8.
Article16. FDA's Sentinel Initiative [Internet]. Silver Spring (MD): U.S. Food and Drug Administration;2017. updated 2016 Dec 14. cited 2017 May 7. Available from: https://www.fda.gov/Safety/FDAsSentinelInitiative/ucm2007250.htm.17. KFDA Developed "Korean Common Data Model". Yonhapnews [Internet]. 2017. 4. 24. cited 2017 May 7. Available from: http://prlink.yonhapnews.co.kr/view.aspx?contents_id=RPR20170424002300353.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Paradigm Shift of Pharmacovigilance: 10 Years’ Experience of Active Surveillance by One Regional Pharmacovigilance Center
- Perspectives of the friction between western and oriental medicines in Korea
- Research Activities on Aerospace Medicine in Japan: Past, Present, and Future
- Growth of North Korean Adolescent Defectors through Temporal Acceptance and Feeling of Security about Traumatic Experience
- Psychosomatic Medicine in Korean Medical Practice : Past, Present and Future