Asia Pac Allergy.
2017 Apr;7(2):82-91. 10.5415/apallergy.2017.7.2.82.
Changes in skin reactivity and associated factors in patients sensitized to house dust mites after 1 year of allergen-specific immunotherapy
- Affiliations
-
- 1Department of Internal Medicine, Kosin University College of Medicine, Busan 49267, Korea. naum67@naver.com
- 2Department of Preventive Medicine, Kosin University College of Medicine, Busan 49267, Korea.
- 3Department of Medical Humanities and Social Medicine, Kosin University College of Medicine, Busan 49267, Korea.
- 4Department of Molecular Biology & Immunology, Kosin University College of Medicine, Busan 49267, Korea.
- KMID: 2396929
- DOI: http://doi.org/10.5415/apallergy.2017.7.2.82
Abstract
- BACKGROUND
Allergen-specific immunotherapy (SIT) can significantly improve symptoms and reduce the need for symptomatic medication.
OBJECTIVE
The aim of this study was to investigate changes in skin reactivity to house dust mites (HDMs) as an immunologic response and associated factors after 1 year of immunotherapy.
METHODS
A total of 80 patients with allergic airway diseases who received subcutaneous SIT with HDMs from 2009 to 2014 were evaluated. The investigated parameters were basic demographic characteristics, skin reactivity and specific IgE for HDM, serum total IgE level, blood eosinophil counts, and medication score.
RESULTS
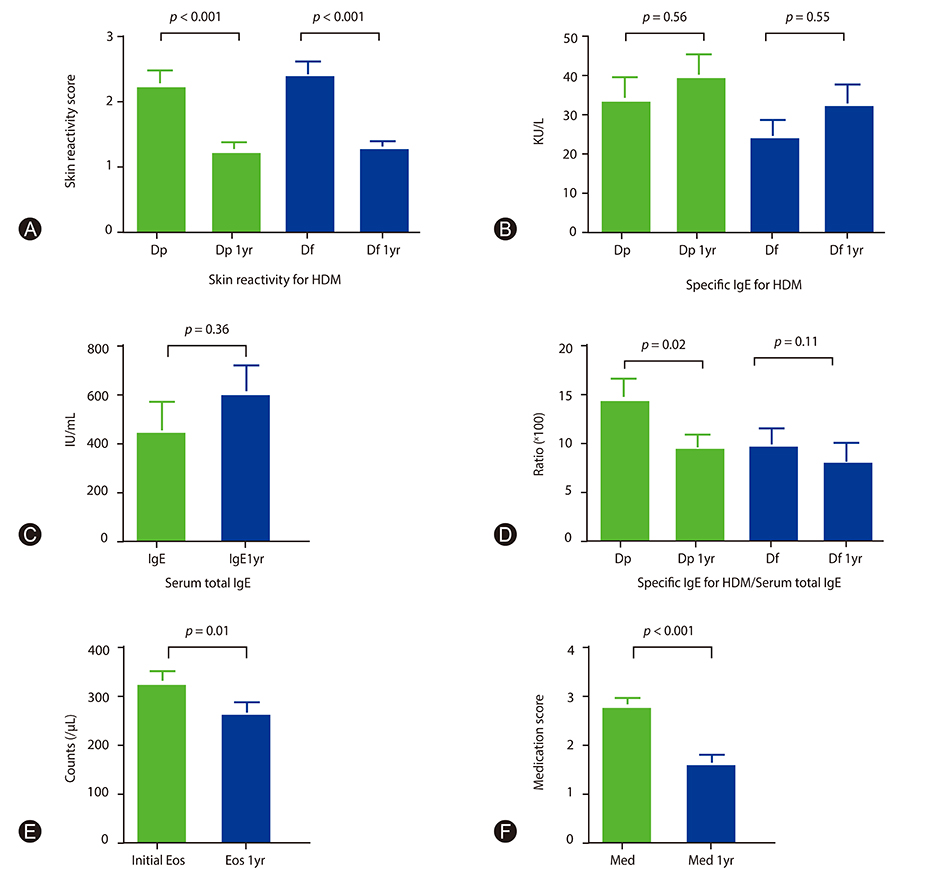
The mean levels of skin reactivity to HDMs, blood eosinophil counts, and medication scores after 1 year were significantly reduced from baseline. In univariate comparison of the changes in skin reactivity to HDMs, age ≤30 years, HDMs only as target of immunotherapy, and high initial skin reactivity (≥2) to HDMs were significantly associated with the reduction in skin test reactivity. In multivariate analysis, high initial skin reactivity and HDMs only as target allergens were significantly associated with changes in skin reactivity to HDMs. In the receiver operating characteristic curve of the initial mean skin reactivity to HDMs for more than 50% reduction, the optimal cutoff value was 2.14.
CONCLUSION
This study showed significant reductions in allergen skin reactivity to HDMs after 1 year of immunotherapy in patients sensitized to HDMs. The extent of initial allergen skin reactivity and only HDMs as target allergen were important predictive factors for changes in skin reactivity.
MeSH Terms
Figure
Reference
-
1. Cox L, Esch RE, Corbett M, Hankin C, Nelson M, Plunkett G. Allergen immunotherapy practice in the United States: guidelines, measures, and outcomes. Ann Allergy Asthma Immunol. 2011; 107:289–299.
Article2. Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, Nelson M, Weber R, Bernstein DI, Blessing-Moore J, Khan DA, Lang DM, Nicklas RA, Oppenheimer J, Portnoy JM, Randolph C, Schuller DE, Spector SL, Tilles S, Wallace D. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011; 127:1 Suppl. S1–S55.3. Jacobsen L, Wahn U, Bilo MB. Allergen-specific immunotherapy provides immediate, long-term and preventive clinical effects in children and adults: the effects of immunotherapy can be categorised by level of benefit -the centenary of allergen specific subcutaneous immunotherapy. Clin Transl Allergy. 2012; 2:8.
Article4. Calderon MA, Casale TB, Nelson HS, Demoly P. An evidence-based analysis of house dust mite allergen immunotherapy: a call for more rigorous clinical studies. J Allergy Clin Immunol. 2013; 132:1322–1336.5. Nanda A, O'connor M, Anand M, Dreskin SC, Zhang L, Hines B, Lane D, Wheat W, Routes JM, Sawyer R, Rosenwasser LJ, Nelson HS. Dose dependence and time course of the immunologic response to administration of standardized cat allergen extract. J Allergy Clin Immunol. 2004; 114:1339–1344.
Article6. Yukselen A, Kendirli SG, Yilmaz M, Altintas DU, Karakoc GB. Effect of one-year subcutaneous and sublingual immunotherapy on clinical and laboratory parameters in children with rhinitis and asthma: a randomized, placebo-controlled, double-blind, double-dummy study. Int Arch Allergy Immunol. 2012; 157:288–298.
Article7. Jutel M, Agache I, Bonini S, Burks AW, Calderon M, Canonica W, Cox L, Demoly P, Frew AJ, O'Hehir R, Kleine-Tebbe J, Muraro A, Lack G, Larenas D, Levin M, Nelson H, Pawankar R, Pfaar O, van Ree R, Sampson H, Santos AF, Du Toit G, Werfel T, Gerth van Wijk R, Zhang L, Akdis CA. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015; 136:556–568.
Article8. Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2007; 119:780–791.
Article9. Larché M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006; 6:761–771.
Article10. Jutel M, Agache I, Bonini S, Burks AW, Calderon M, Canonica W, Cox L, Demoly P, Frew AJ, O'Hehir R, Kleine-Tebbe J, Muraro A, Lack G, Larenas D, Levin M, Martin BL, Nelson H, Pawankar R, Pfaar O, van Ree R, Sampson H, Sublett JL, Sugita K, Du Toit G, Werfel T, Gerth van Wijk R, Zhang L, Akdis M, Akdis CA. International Consensus on Allergen Immunotherapy II: Mechanisms, standardization, and pharmacoeconomics. J Allergy Clin Immunol. 2016; 137:358–368.
Article11. Tonnel AB, Scherpereel A, Douay B, Mellin B, Leprince D, Goldstein N, Delecluse P, Andre C. Allergic rhinitis due to house dust mites: evaluation of the efficacy of specific sublingual immunotherapy. Allergy. 2004; 59:491–497.
Article12. Cingi C, Bayar Muluk N, Ulusoy S, Acar M, Şirin S, Çobanoğlu B, Birdane L, Kalaycık Ç, Çakır BÖ, Oğhan F, Aynacı S, Erdoğmuş N, Yıldırım Ö, Şahin E, Bulut F, Aksoy MA, Türe N, Bal C. Efficacy of sublingual immunotherapy for house dust mite allergic rhinitis. Eur Arch Otorhinolaryngol. 2015; 272:3341–3346.
Article13. Kim ST. Outcome of sublingual immunotherapy in patients with allergic rhinitis sensitive to house dust mites. Allergy Asthma Immunol Res. 2015; 7:99–100.
Article14. Kim SH, Shin SY, Lee KH, Kim SW, Cho JS. Long-term effects of specific allergen immunotherapy against house dust mites in polysensitized patients with allergic rhinitis. Allergy Asthma Immunol Res. 2014; 6:535–540.
Article15. Soyyigit S, Guloglu D, Ikinciogullari A, Secil D, Oztuna D, Mungan D, Misirligil Z, Sin BA. Immunologic alterations and efficacy of subcutaneous immunotherapy with Dermatophagoides pteronyssinus in monosensitized and polysensitized patients. Ann Allergy Asthma Immunol. 2016; 116:244–251.e2.
Article16. Kim KW, Kim EA, Kwon BC, Kim ES, Song TW, Sohn MH, Kim KE. Comparison of allergic indices in monosensitized and polysensitized patients with childhood asthma. J Korean Med Sci. 2006; 21:1012–1016.
Article17. Calderón MA, Cox L, Casale TB, Moingeon P, Demoly P. Multiple-allergen and single-allergen immunotherapy strategies in polysensitized patients: looking at the published evidence. J Allergy Clin Immunol. 2012; 129:929–934.
Article18. Nelson HS. Specific immunotherapy with allergen mixes: what is the evidence? Curr Opin Allergy Clin Immunol. 2009; 9:549–553.
Article19. Panizo C, Cimarra M, González-Mancebo E, Vega A, Senent C, Martín S. In vivo and in vitro immunological changes induced by a short course of grass allergy immunotherapy tablets. J Investig Allergol Clin Immunol. 2010; 20:454–462.20. Lee E, Kim MJ, Yang SI, Yu J, Hong SJ. Comparison of short-term effects between subcutaneous and sublingual immunotherapies in children with house dust mite-sensitized allergic rhinitis and asthma. Allergy Asthma Respir Dis. 2015; 3:180–186.
Article21. Kim JH, Jang AS, Jeong SO, Ji YS, Seo HJ, Nam JH, Moon JJ, Baek AR, Park JS, Lee JH, Park SW, Kim DJ, Park CS. The differences of clinical profiles by house dust mite sensitization in patients with asthmatics in Soonchunhyang University Hospital cohort. Allergy Asthma Respir Dis. 2013; 1:50–54.
Article22. Jeong KY, Park JW, Hong CS. House Dust Mite Allergy in Korea: The Most Important Inhalant Allergen in Current and Future. Allergy Asthma Immunol Res. 2012; 4:313–325.
Article23. Park HJ, Lim HS, Park KH, Lee JH, Park JW, Hong CS. Changes in allergen sensitization over the last 30 years in korea respiratory allergic patients: a single-center. Allergy Asthma Immunol Res. 2014; 6:434–443.
Article24. Lee JE, Ahn JC, Han DH, Kim DY, Kim JW, Cho SH, Park HW, Rhee CS. Variability of offending allergens of allergic rhinitis according to age: optimization of skin prick test allergens. Allergy Asthma Immunol Res. 2014; 6:47–54.
Article25. Hur GY, Kim TB, Han MY, Nahm DH, Park JW. Allergen and Immunotherapy Work Group of the Korean Academy of Asthma. Allergy and Clinical Immunology (KAAACI). A survey of the prescription patterns of allergen immunotherapy in Korea. Allergy Asthma Immunol Res. 2013; 5:277–282.
Article26. Kim BS, Lee SK, Park HS, Hong SJ. The early changes of humoral immune response after rush immunotherapy with Dermatophagoides farinae (D.f) and Dermatophagoides pteronyssinus (D.p) in house dust mite sensitive asthmatic children. J Asthma Allergy Clin Immunol. 2001; 21:543–551.27. Cevit O, Kendirli SG, Yilmaz M, Altintas DU, Karakoc GB. Specific allergen immunotherapy: effect on immunologic markers and clinical parameters in asthmatic children. J Investig Allergol Clin Immunol. 2007; 17:286–291.28. Aasbjerg K, Backer V, Lund G, Holm J, Nielsen NC, Holse M, Wagtmann VR, Würtzen PA. Immunological comparison of allergen immunotherapy tablet treatment and subcutaneous immunotherapy against grass allergy. Clin Exp Allergy. 2014; 44:417–428.
Article29. Eifan AO, Akkoc T, Yildiz A, Keles S, Ozdemir C, Bahceciler NN, Barlan IB. Clinical efficacy and immunological mechanisms of sublingual and subcutaneous immunotherapy in asthmatic/rhinitis children sensitized to house dust mite: an open randomized controlled trial. Clin Exp Allergy. 2010; 40:922–932.
Article30. Kim HB, Lee SY, Kim JH, Kim BS, Seo HJ, Hong SJ. A Three-year Follow-up of D.f- and D.p-Specific Conventional Immunotherapy in Asthmatic Children. Pediatr Allergy Respir Dis. 2005; 15:26–34.31. Li Q, Li M, Yue W, Zhou J, Li R, Lin J, Li Y. Predictive factors for clinical response to allergy immunotherapy in children with asthma and rhinitis. Int Arch Allergy Immunol. 2014; 164:210–217.
Article32. Peng H, Li CW, Lin ZB, Li TY. Long-term efficacy of specific immunotherapy on house dust mite-induced allergic rhinitis in China. Otolaryngol Head Neck Surg. 2013; 149:40–46.
Article33. Löfkvist T, Agrell B, Dreborg S, Svensson G. Effects of immunotherapy with a purified standardized allergen preparation of Dermatophagoides farinae in adults with perennial allergic rhinoconjunctivitis. Allergy. 1994; 49:100–107.
Article34. Karakoc-Aydiner E, Eifan AO, Baris S, Gunay E, Akturk E, Akkoc T, Bahceciler NN, Barlan IB. Long-term effect of sublingual and subcutaneous immunotherapy in dust mite-allergic children with asthma/rhinitis: a 3-year prospective randomized controlled trial. J Investig Allergol Clin Immunol. 2015; 25:334–342.35. Pajno GB, Barberio G, De Luca F, Morabito L, Parmiani S. Prevention of new sensitizations in asthmatic children monosensitized to house dust mite by specific immunotherapy. A six-year follow-up study. Clin Exp Allergy. 2001; 31:1392–1397.
Article36. Li L, Guan K. Effect on quality of life of the mixed house dust mite/weed pollen extract immunotherapy. Asia Pac Allergy. 2016; 6:168–173.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Influence of Conventional Immunotherapy with D.f and D.p on Eosinophil, Specific IgE, Skin Test Reactivity, and Airway Hyperreactivity in House Dust Mite Sensitive Asthmatic Children
- House Dust Mites Sensitivity in Korean Atopic children; Correlation Between Skin Reaction and Relative Concentration of Specific IgE by ELISA
- Treatment of Patients with Refractory Atopic Dermatitis Sensitized to House Dust Mites by Using Sublingual Allergen Immunotherapy
- The early changes of humoral immune response after rush immunotherapy with Dermatophagoides farinae (D.f) and Dermatophagoides pteronyssinus (D.p) in house dust mite sensitive asthmatic children
- Two Cases of Atopic Dermatitis Improved by Combination Treatment of Allergen-Specific Immunotherapy and Histamine-Immunoglobulin Complex