Ann Surg Treat Res.
2017 Dec;93(6):316-321. 10.4174/astr.2017.93.6.316.
Efficacy and safety of a novel partially absorbable mesh in totally extraperitoneal hernia repair
- Affiliations
-
- 1Department of Surgery, Korea University Guro Hospital, Seoul, Korea. silee@korea.ac.kr
- KMID: 2396873
- DOI: http://doi.org/10.4174/astr.2017.93.6.316
Abstract
- PURPOSE
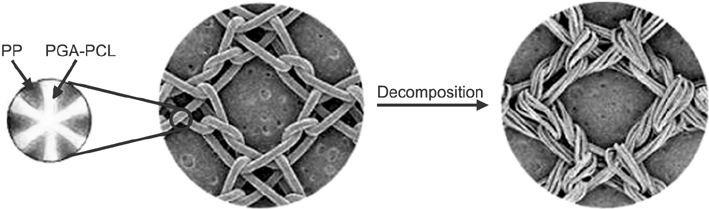
Partially absorbable mesh has been introduced and used for inguinal hernia repair for the purpose of minimizing pain and improving abdominal wall compliance. In this study, we evaluate the efficacy and safety of ProFlex mesh, a partially absorbed mesh with new structural architecture.
METHODS
We retrospectively reviewed 64 cases of totally extraperitoneal herniorrhapy (TEP) from January 2013 to December 2014 for their clinical features, including operation time, pain, postoperative complications, and recurrence.
RESULTS
There were no significant differences in operation time, hospital stay, postoperative pain, or complications between the 28 patients who received the ProFlex mesh and the 36 who received nonabsorbable lightweight mesh, although one patient who received the nonabsorbable had a recurrence during follow-up. There were differences in operation time, complications, and hospital stay according to the surgeon's previous operation volume.
CONCLUSION
This study showed that there were significant differences in the fixation strength of different polypropylene meshes in combination with various fibrin glues. ProFlex, a partially absorbable mesh with new architecture, was feasible and safe in TEP.
Keyword
MeSH Terms
Figure
Reference
-
1. Bay-Nielsen M, Kehlet H, Strand L, Malmstrom J, Andersen FH, Wara P, et al. Quality assessment of 26,304 herniorrhaphies in Denmark: a prospective nationwide study. Lancet. 2001; 358:1124–1128.2. EU Hernia Trialists Collaboration. Repair of groin hernia with synthetic mesh: meta-analysis of randomized controlled trials. Ann Surg. 2002; 235:322–332.3. van Veen RN, Wijsmuller AR, Vrijland WW, Hop WC, Lange JF, Jeekel J. Long-term follow-up of a randomized clinical trial of non-mesh versus mesh repair of primary inguinal hernia. Br J Surg. 2007; 94:506–510.4. Klinge U, Klosterhalfen B, Müller M, Schumpelick V. Foreign body reaction to meshes used for the repair of abdominal wall hernias. Eur J Surg. 1999; 165:665–673.5. Cunningham J, Temple WJ, Mitchell P, Nixon JA, Preshaw RM, Hagen NA. Cooperative hernia study. Pain in the postrepair patient. Ann Surg. 1996; 224:598–602.6. Poobalan AS, Bruce J, King PM, Chambers WA, Krukowski ZH, Smith WC. Chronic pain and quality of life following open inguinal hernia repair. Br J Surg. 2001; 88:1122–1126.7. Post S, Weiss B, Willer M, Neufang T, Lorenz D. Randomized clinical trial of lightweight composite mesh for Lichtenstein inguinal hernia repair. Br J Surg. 2004; 91:44–48.8. O'Dwyer PJ, Kingsnorth AN, Molloy RG, Small PK, Lammers B, Horeyseck G. Randomized clinical trial assessing impact of a lightweight or heavyweight mesh on chronic pain after inguinal hernia repair. Br J Surg. 2005; 92:166–170.9. Bringman S, Wollert S, Osterberg J, Smedberg S, Granlund H, Heikkinen TJ. Three-year results of a randomized clinical trial of lightweight or standard polypropylene mesh in Lichtenstein repair of primary inguinal hernia. Br J Surg. 2006; 93:1056–1059.10. Paajanen H. A single-surgeon randomized trial comparing three composite meshes on chronic pain after Lichtenstein hernia repair in local anesthesia. Hernia. 2007; 11:335–339.11. Koch A, Bringman S, Myrelid P, Smeds S, Kald A. Randomized clinical trial of groin hernia repair with titanium-coated lightweight mesh compared with standard polypropylene mesh. Br J Surg. 2008; 95:1226–1231.12. Polish Hernia Study Group. smietanski M. Randomized clinical trial comparing a polypropylene with a poliglecaprone and polypropylene composite mesh for inguinal hernioplasty. Br J Surg. 2008; 95:1462–1468.13. Nikkolo C, Lepner U, Murruste M, Vaasna T, Seepter H, Tikk T. Randomised clinical trial comparing lightweight mesh with heavyweight mesh for inguinal hernioplasty. Hernia. 2010; 14:253–258.14. Bringman S, Wol lert S, Osterberg J, Heikkinen T. Early results of a randomized multicenter trial comparing Prolene and VyproII mesh in bilateral endoscopic extraperitoneal hernioplasty (TEP). Surg Endosc. 2005; 19:536–540.15. Heikkinen T, Wollert S, Osterberg J, Smedberg S, Bringman S. Early results of a randomised tr ial compar ing Prolene and VyproII-mesh in endoscopic extraperitoneal inguinal hernia repair (TEP) of recurrent unilateral hernias. Hernia. 2006; 10:34–40.16. Langenbach MR, Schmidt J, Zirngibl H. Comparison of biomaterials: three meshes and TAPP for inguinal hernia. Surg Endosc. 2006; 20:1511–1517.17. Agarwal BB, Agarwal KA, Mahajan KC. Prospective doubleblind randomized controlled study comparing heavy- and lightweight polypropylene mesh in totally extraperitoneal repair of inguinal hernia: early results. Surg Endosc. 2009; 23:242–247.18. Chui LB, Ng WT, Sze YS, Yuen KS, Wong YT, Kong CK. Prospective, randomized, controlled trial comparing lightweight versus heavyweight mesh in chronic pain incidence after TEP repair of bilateral inguinal hernia. Surg Endosc. 2010; 24:2735–2738.19. Chowbey PK, Garg N, Sharma A, Khullar R, Soni V, Baijal M, et al. Prospective randomized clinical trial comparing lightweight mesh and heavyweight polypropylene mesh in endoscopic totally extraperitoneal groin hernia repair. Surg Endosc. 2010; 24:3073–3079.20. Bittner R, Schmedt CG, Leibl BJ, Schwarz J. Early postoperative and one year results of a randomized controlled trial comparing the impact of extralight titanized polypropylene mesh and traditional heavyweight polypropylene mesh on pain and seroma production in laparoscopic hernia repair (TAPP). World J Surg. 2011; 35:1791–1797.21. Cristaudo A, Nayak A, Martin S, Adib R, Martin I. A prospective randomised trial comparing mesh types and fixation in totally extraperitoneal inguinal hernia repairs. Int J Surg. 2015; 17:79–82.22. Currie A, Andrew H, Tonsi A, Hurley PR, Taribagil S. Lightweight versus heavyweight mesh in laparoscopic inguinal hernia repair: a metaanalysis. Surg Endosc. 2012; 26:2126–2133.23. Gao D, Wei S, Zhai C, Chen J, Li M, Gu C, et al. Clinical research of preperitoneal drainage af ter endoscopic total ly extraperitoneal inguinal hernia repair. Hernia. 2015; 19:789–794.24. Burgmans JP, Voorbrood CE, Schouten N, Smakman N, Elias S, Clevers GJ, et al. Threemonth results of the effect of Ultrapro or Prolene mesh on post-operative pain and well-being following endoscopic totally extraperitoneal hernia repair (TULP trial). Surg Endosc. 2015; 29:3171–3178.25. Ujiki MB, Gitelis ME, Carbray J, Lapin B, Linn J, Haggerty S, et al. Patient-centered outcomes following laparoscopic inguinal hernia repair. Surg Endosc. 2015; 29:2512–2519.26. Bittner R, Leibl BJ, Jäger C, Kraft B, Ulrich M, Schwarz J. TAPP Stuttgart technique and result of a large single center series. J Minim Access Surg. 2006; 2:155–159.27. Bittner R, Gmähle E, Gmähle B, Schwarz J, Aasvang E, Kehlet H. Lightweight mesh and noninvasive fixation: an effective concept for prevention of chronic pain with laparoscopic hernia repair (TAPP). Surg Endosc. 2010; 24:2958–2964.28. Bittner R, Arregui ME, Bisgaard T, Dudai M, Ferzli GS, Fitzgibbons RJ, et al. Guidelines for laparoscopic (TAPP) and endoscopic (TEP) treatment of inguinal hernia [International Endohernia Society (IEHS)]. Surg Endosc. 2011; 25:2773–2843.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Totally Extraperitoneal Laparoscopic Repair of Obturator Hernia withPartial Intestinal Obstruction
- Laparoscopic total extraperitoneal repair of lumbar hernia
- Prevention and management of intraoperative complication during single incision laparoscopic totally extraperitoneal repair
- To Minimize Post-operative Pain in Inguinal Hernia Repair: Single-port Laparoscopic Totally Extraperitoneal Inguinal Hernia Repair without Fixation of the Mesh
- Management of Infected Mesh after Laparoscopic Incisional Hernia Repair