Ann Dermatol.
2017 Dec;29(6):768-775. 10.5021/ad.2017.29.6.768.
Serologic Response to Treatment in Human Immunodeficiency Virus-Negative Syphilis Patients Using Automated Serological Tests: Proposals for New Guidelines
- Affiliations
-
- 1Department of Dermatology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea. gylee0716@hanmail.net, susini@naver.com
- KMID: 2395186
- DOI: http://doi.org/10.5021/ad.2017.29.6.768
Abstract
- BACKGROUND
Automated analyzer-based nontreponemal serological tests for syphilis (STS) have been used for several decades.
OBJECTIVE
In this study, we evaluated serological responses to treatment and proposed clinical guidelines for automated STS.
METHODS
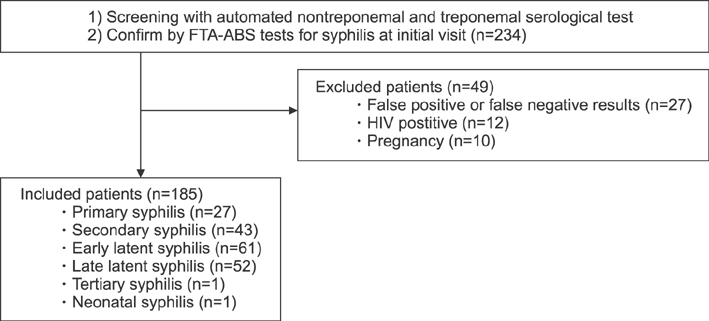
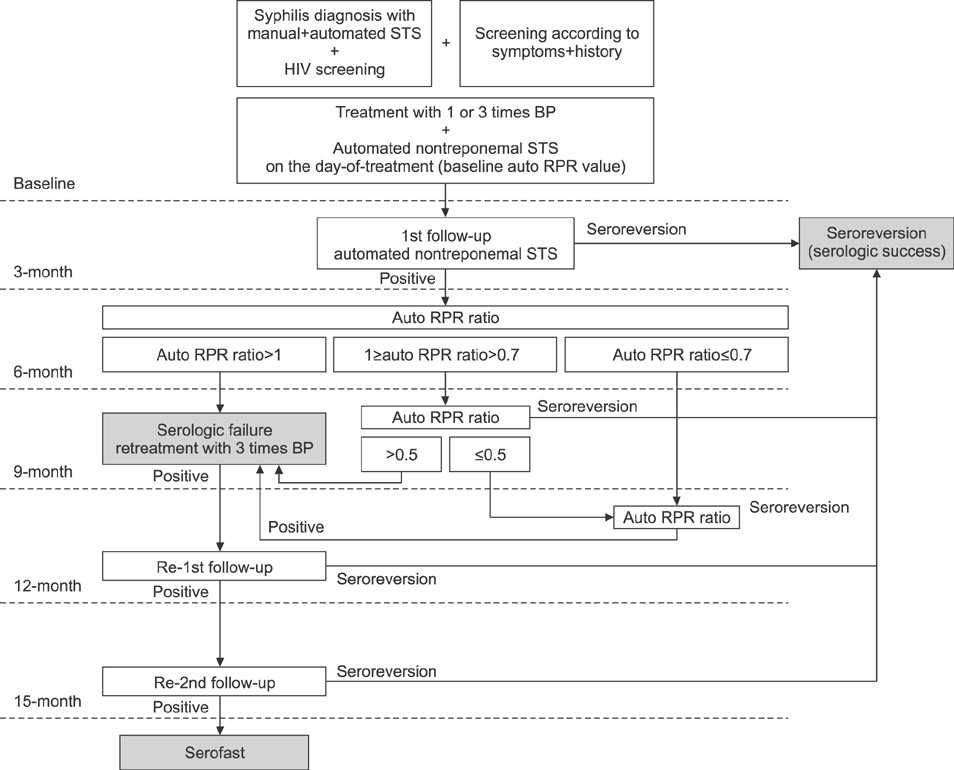
This retrospective cohort study analyzed human immunodeficiency virus-negative syphilis patients who were diagnosed with automated rapid plasma reagin (auto RPR) tests as a nontreponemal STS, and who also received the fluorescent treponemal antibody-absorption test as a confirmatory test. The ratio of auto RPR values after treatment against those at baseline was defined as the auto RPR ratio for the analysis of the serological response to treatment. The cutoff value for reliable seroreversion prediction was assessed with receiver-operating-characteristic curves.
RESULTS
Overall, 89.7% of participants (78/87) seroreverted and 10.3% of participants (9/87) remained serofast during the two-year follow-up period. We were unable to describe trends in the changes among auto RPR values within six months after treatment because of high variation. All of the patients who had an auto RPR ratio ≥1.0 after six months continuously had positive serologic results during their 24-month follow-up and were classified as a serofast group. The receiver-operating-characteristic curves revealed a 25% reduction in auto RPR values nine months after treatment and predicted seroreversion with a sensitivity of 96.2% and a specificity of 100%.
CONCLUSION
The most important primary checkpoint for syphilis treatment response is an increase in automated nontreponemal STS six months after treatment. Thus, we recommend monitoring the treatment response with an auto RPR.
Keyword
MeSH Terms
Figure
Reference
-
1. Kampmeier RH. The introduction of penicillin for the treatment of syphilis. Sex Transm Dis. 1981; 8:260–265.
Article2. Workowski KA, Berman S. Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010; 59:1–110.3. Janier M, Hegyi V, Dupin N, Unemo M, Tiplica GS, Potočnik M, et al. 2014 European guideline on the management of syphilis. J Eur Acad Dermatol Venereol. 2014; 28:1581–1593.
Article4. Brown ST, Zaidi A, Larsen SA, Reynolds GH. Serological response to syphilis treatment. A new analysis of old data. JAMA. 1985; 253:1296–1299.
Article5. Müller I, Brade V, Hagedorn HJ, Straube E, Schörner C, Frosch M, et al. Is serological testing a reliable tool in laboratory diagnosis of syphilis? Meta-analysis of eight external quality control surveys performed by the german infection serology proficiency testing program. J Clin Microbiol. 2006; 44:1335–1341.
Article6. Choi SJ, Park Y, Lee EY, Kim S, Kim HS. Comparisons of fully automated syphilis tests with conventional VDRL and FTA-ABS tests. Clin Biochem. 2013; 46:834–837.
Article7. Onoe T, Honda M, Matsuo K, Sasaki H, Sawamura M, Onoe Y, et al. Examination of the correlation between the manual and automated serological testing methods for syphilis. J Dermatol. 2012; 39:355–361.
Article8. Wang HC, Chen C, Wang LN, Long YF, Zhang WZ, Li YQ, et al. The clinical significance comparison of a latex agglutination based syphilis screening test at low antibody titer. Clin Lab. 2013; 59:1429–1432.
Article9. Liu C, Ou Q, Chen H, Chen J, Lin S, Jiang L, et al. The diagnostic value and performance evaluation of five serological tests for the detection of Treponema pallidum. J Clin Lab Anal. 2014; 28:204–209.
Article10. Lee JH, Lim CS, Lee MG, Kim HS. Comparison of an automated rapid plasma reagin (RPR) test with the conventional RPR card test in syphilis testing. BMJ Open. 2014; 4:e005664.
Article11. Case definitions for infectious conditions under public health surveillance. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1997; 46:1–55.12. Seña AC, Wolff M, Martin DH, Behets F, Van Damme K, Leone P, et al. Predictors of serological cure and Serofast State after treatment in HIV-negative persons with early syphilis. Clin Infect Dis. 2011; 53:1092–1099.
Article13. Holman KM, Wolff M, Seña AC, Martin DH, Behets F, Van Damme K, et al. Rapid plasma reagin titer variation in the 2 weeks after syphilis therapy. Sex Transm Dis. 2012; 39:645–647.
Article14. Knaute DF, Graf N, Lautenschlager S, Weber R, Bosshard PP. Serological response to treatment of syphilis according to disease stage and HIV status. Clin Infect Dis. 2012; 55:1615–1622.
Article15. Rolfs RT, Joesoef MR, Hendershot EF, Rompalo AM, Augenbraun MH, Chiu M, et al. A randomized trial of enhanced therapy for early syphilis in patients with and without human immunodeficiency virus infection. The Syphilis and HIV study group. N Engl J Med. 1997; 337:307–314.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- New Developments in the Immunological Understanding and of Serodiagnosis in Syphilis
- Two Cases of Unusual Manifestations of Secondary Syphilis Accompanied by Human Immunodeficiency Virus Infection
- Survey on Serological Test for Syphilis in Korea Healthy Youths
- Interpretation of Serological Tests for Latent Syphilis
- A Case of Acquired Syphilitic Osteomyelitis of the Tibia