J Dent Rehabil Appl Sci.
2017 Sep;33(3):178-188. 10.14368/jdras.2017.33.3.178.
Analysis of temperature changes and sterilization effect of diode laser for the treatment of peri-implantitis by wavelength and irradiation time
- Affiliations
-
- 1Department of Prosthodontics, School of Dentistry, Seoul National University, Seoul, Republic of Korea. limdds@snu.ac.kr
- 2Department of Conservative Dentistry and Dental Research Institute, Seoul National University, Seoul, Republic of Korea.
- 3Department of Oral and Maxillofacial Surgery, Clinical Trial Center, Seoul National University, Seoul, Republic of Korea.
- KMID: 2394641
- DOI: http://doi.org/10.14368/jdras.2017.33.3.178
Abstract
- PURPOSE
We compared the effects of newly developed diode laser (Bison 808 nm Diode laser) on the treatment of peri-implantitis with conventional products (Picasso 810 nm Diode laser) by comparing the surface temperature of titanium disc and bacterial sterilization according to laser power.
MATERIALS AND METHODS
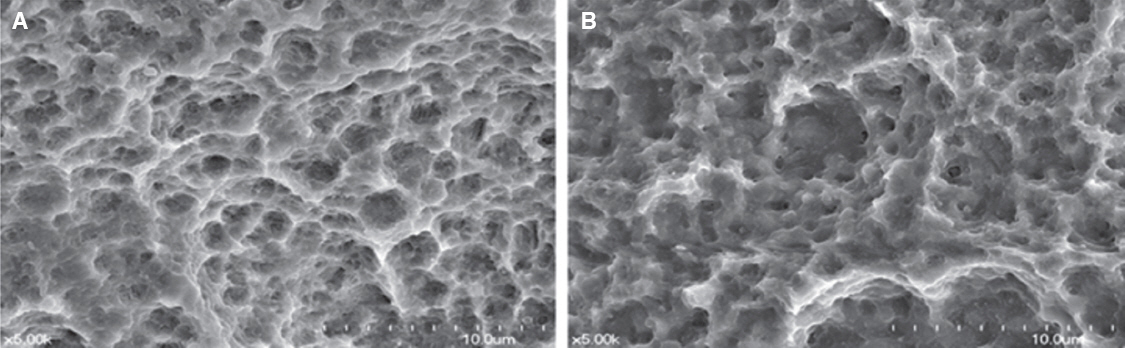
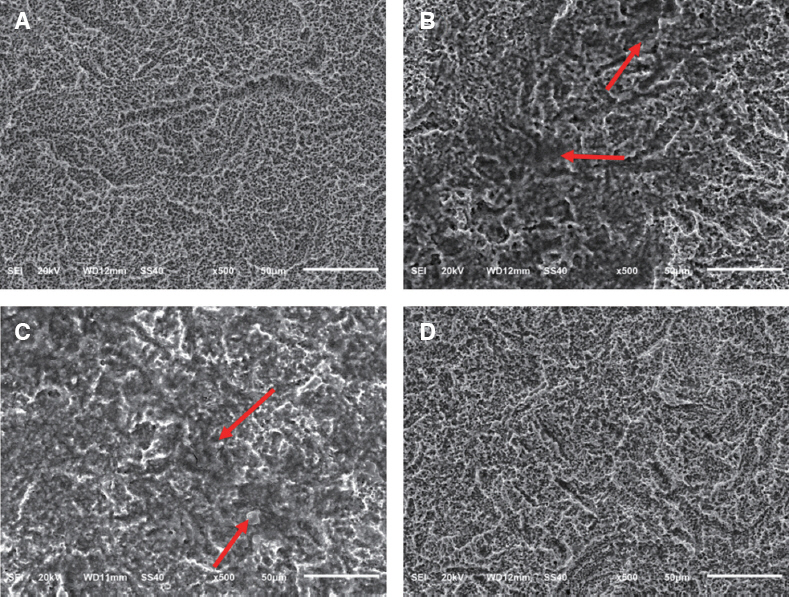
The titanium disc was irradiated for 60 seconds and 1 - 2.5 W using diode laser 808 nm and 810 nm. The surface temperature of the titanium disc was measured using a temperature measurement module and a temperature measurement program. In addition, in order to investigate the sterilizing effect according to the laser power, 808 nm laser was irradiated after application of bacteria to sandblasted large-grit acid-etched (SLA) and resorbable blast media (RBM) coated titanium discs. The irradiated disks were examined with scanning electron microscopy.
RESULTS
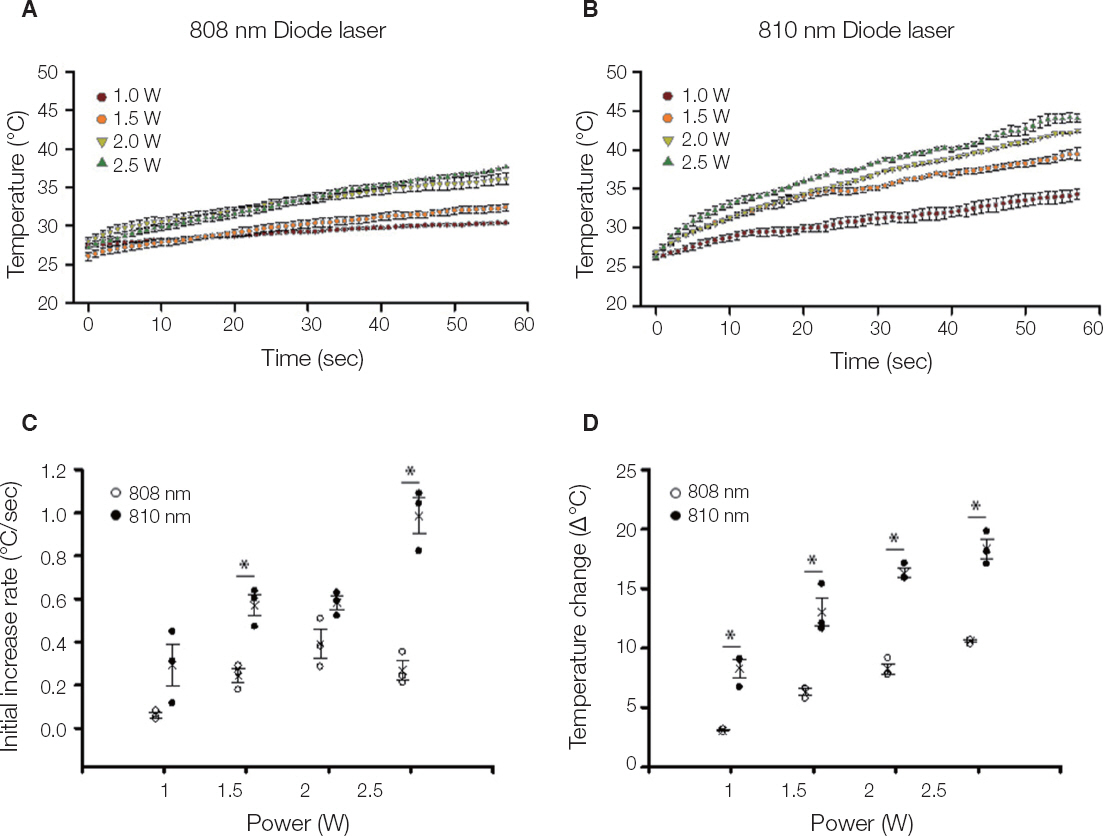
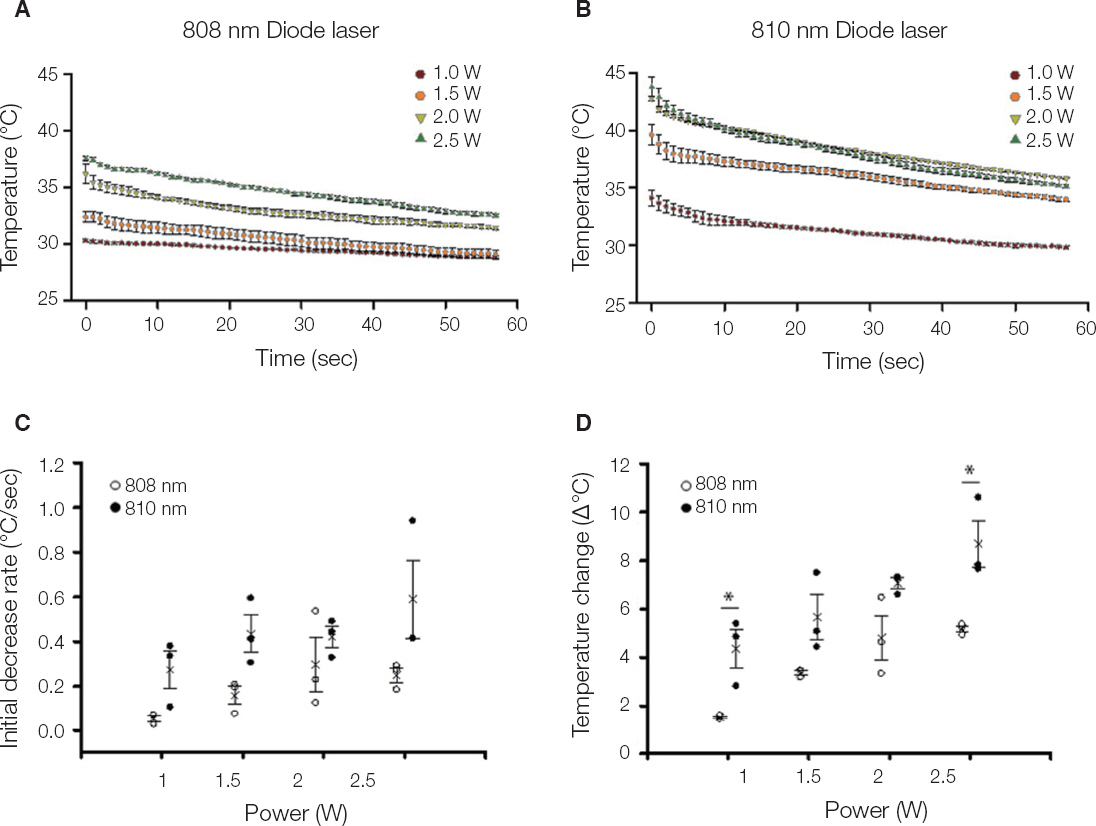
Both 808 nm and 810 nm lasers increased disk surface temperature as the power increased. When the 810 nm was irradiated under all conditions, the initial temperature rise rate, the descending rate, and the temperature change before and after was higher than that of 808 nm. Disk surface changes were not observed on both lasers at all conditions. Bacteria were irradiated with 808 nm, and the bactericidal effect was increased as the power increased.
CONCLUSION
When applying these diode lasers to the treatment of peri-implantitis, 808 nm which have a bactericidal effect with less temperature fluctuation in the same power conditions would be considered safer. However, in order to apply a laser treatment in the dental clinical field, various safety and reliability should be secured.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Ishikawa I, Aoki A, Takasaki AA, Mizutani K, Sasaki KM, Izumi Y. Application of lasers in periodontics: true innovation or myth? Periodontol 2000. 2009; 50:90–126. DOI: 10.1111/j.1600-0757.2008.00283.x. PMID: 19388956.2. Dörtbudak O, Haas R, Bernhart T, Mailath-Pokorny G. Lethal photosensitization for decontamination of implant surfaces in the treatment of periimplantitis. Clin Oral Implants Res. 2001; 12:104–8. DOI: 10.1034/j.1600-0501.2001.012002104.x. PMID: 11251658.3. Schwarz F, Sculean A, Rothamel D, Schwenzer K, Georg T, Becker J. Clinical evaluation of an Er: YAG laser for nonsurgical treatment of periimplantitis: a pilot study. Clin Oral Implants Res. 2005; 16:44–52. DOI: 10.1111/j.1600-0501.2004.01051.x. PMID: 15642030.4. Atieh MA, Alsabeeha NH, Faggion CM Jr, Duncan WJ. The frequency of peri-implant diseases: a systematic review and meta-analysis. J Periodontol. 2013; 84:1586–98. PMID: 23237585.5. Lindhe J, Meyle J; Group D of European Workshop on Periodontology. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008; 35:282–5. DOI: 10.1111/j.1600-051X.2008.01283.x. PMID: 18724855.6. Froum SJ, Rosen PS. A proposed classification for peri-implantitis. Int J Periodontics Restorative Dent. 2012; 32:533–40. PMID: 22754901.7. Esposito M, Grusovin MG, Kakisis I, Coulthard P, Worthington HV. Interventions for replacing missing teeth: treatment of peri implantitis. Cochrane Database Syst Rev. 2008; Apr. 16(2):CD004970. doi:10.1002/14651858.CD004970.pub3. DOI: 10.1002/14651858.CD004970.pub3.8. Ntrouka VI, Slot DE, Louropoulou A, Van der Weijden F. The effect of chemotherapeutic agents on contaminated titanium surfaces: a systematic review. Clin Oral Implants Res. 2011; 22:681–90. DOI: 10.1111/j.1600-0501.2010.02085.x. PMID: 21198891.9. Kotsovilis S, Karoussis IK, Trianti M, Fourmousis I. Therapy of peri-implantitis: a systematic review. J Clin Periodontol. 2008; 35:621–9. DOI: 10.1111/j.1600-051X.2008.01240.x. PMID: 18476998.10. Tosun E, Tasar F, Strauss R, Kivanc DG, Ungor C. Comparative evaluation of antimicrobial effects of Er: YAG, diode, and CO2 lasers on titanium discs: and experimental study. J Oral Maxillofac Surg. 2012; 70:1064–9. DOI: 10.1016/j.joms.2011.11.021. PMID: 22285338.11. Stubinger S, Etter C, Miskiewicz M, Homann F, Saldamli B, Wieland M, Sader R. Surface alterations of polished and sandblasted and acid-etched titanium implants after Er: YAG, carbon dioxide, and diode laser irradiation. Int J Oral Maxillofac Implants. 2010; 25:104–11. PMID: 20209192.12. Park JH, Heo SJ, Koak JY, Kim SK, Han CH, Lee JH. Effects of laser irradiation on machined and anodized titanium disks. Int J Oral Maxillofac Implants. 2012; 27:265–72. PMID: 22442763.13. Kreisler M, Al Haj H, Götz H, Duschner H, d'Hoedt B. Effect of simulated CO2 and GaAlAs laser surface decontamination on temperature changes in Ti-Plasma sprayed dental implants. Lasers Surg Med. 2002; 30:233–9. DOI: 10.1002/lsm.10025. PMID: 11891744.14. Kim JH, Choi BK, Choi SH, Cho KS, Chai JK, Kim CK. Ribotyping of Porphyromonas Gingivalis isolated from rapidly progressive periodontitis patients. J Korean Acad Periodontol. 1999; 29:963–77. DOI: 10.5051/jkape.1999.29.4.963.15. Kim SY, Woo DH, Lee MA, Kim JS, Lee JH, Jeong SH. Red fluorescence of oral bacteria interacting with Porphyromonas gingivalis. J Korean Acad Oral Health. 2017; 41:22–7. DOI: 10.11149/jkaoh.2017.41.1.22.16. Peck SY, Ku Y, Rhyu IC, Hahm BD, Han SB, Choi SM, Chung CP. The frequency of detecting Prevotella intermedia and Prevotella nigrescens in Korean adult periodontitis patients. J Korean Acad Periodontol. 2000; 30:419–27. DOI: 10.5051/jkape.2000.30.2.419.17. Romanos GE, Everts H, Nentwig GH. Effects of diode and Nd: YAG laser irradiation of titanium discs: a scanning electron microscope examination. J Periodontol. 2000; 71:810–5. DOI: 10.1902/jop.2000.71.5.810. PMID: 10872964.18. Slater SC, Voige WH, Dennis DE. Cloning and expression in Escherichia coli of the Alcaligenes eutrophus H16 poly-beta-hydroxybutyrate biosynthetic pathway. J Bacteriol. 1988; 170:4431–6. DOI: 10.1128/jb.170.10.4431-4436.1988. PMID: 3049530. PMCID: PMC211473.19. McDaniel TK, Kaper JB. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K 12. Mol Microbiol. 1997; 23:399–407. DOI: 10.1046/j.1365-2958.1997.2311591.x. PMID: 9044273.20. Cooper GM. The cell: a molecular approach. 4th ed. Sunderland: Sinauer Associates, Inc;2007. p. 43–73. PMID: 18368739.21. Lee KJ, Lee JH, Kum KY, Lim YJ. Laser therapy in peri-implantitis treatment: literature review. J Dent Rehabil Appl Sci. 2015; 31:340–8. DOI: 10.14368/jdras.2015.31.4.340.22. Kreisler M, Kohnen W, Marinello C, Götz H, Duschner H, Jansen B, D'Hoedt B. Bactericidal effect of the Er: YAG laser on dental implant surfaces: an in vitro study. J Periodontol. 2002; 73:1292–8. DOI: 10.1902/jop.2002.73.11.1292. PMID: 12479633.23. Koo PM, Lee DW. The diagnosis and treatment of peri-implantitis. J Dent Implant Res. 2015; 34:76–80.24. Oh WS, Ryu JJ, Ji S. Microbiology of Peri-implantitis. J Dent Implant Res. 2013; 32:1–6.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Laser therapy in peri-implantitis treatment: literature review
- Scanning electron microscopic study of implant surface after Er,Cr:YSGG laser irradiation
- Effect of Photothermal Therapy with Indocyanine Green in Multispecies Biofilm
- Er: YAG Laser Irradiated Implant Surface Observation with Scanning Electron Microscopy
- Evaluation of the effectiveness of diode laser therapy in conjunction with nonsurgical treatment of periimplantitis