Nutr Res Pract.
2016 Aug;10(4):393-397. 10.4162/nrp.2016.10.4.393.
Therapeutic effects of dihydroartemisinin and transferrin against glioblastoma
- Affiliations
-
- 1Department of Food and Nutrition, Hyejeon College, Chungnam 32244, Korea.
- 2Department of Radiological Science, College of Medical Science, Konyang University, 158 Gwanjeodong-ro, Seo-gu, Daejeon 35365, Korea. bskang@konyang.ac.kr
- KMID: 2390141
- DOI: http://doi.org/10.4162/nrp.2016.10.4.393
Abstract
- BACKGROUND/OBJECTIVES
Artemisinin, a natural product isolated from Gaeddongssuk (artemisia annua L.) and its main active derivative, dihydroartemisinin (DHA), have long been used as antimalarial drugs. Recent studies reported that artemisinin is efficacious for curing diseases, including cancers, and for improving the immune system. Many researchers have shown the therapeutic effects of artemisinin on tumors such as breast cancer, liver cancer and kidney cancer, but there is still insufficient data regarding glioblastoma (GBM). Glioblastoma accounts for 12-15% of brain cancer, and the median survival is less than a year, despite medical treatments such as surgery, radiation therapy, and chemotherapy. In this study, we investigated the anti-cancer effects of DHA and transferrin against glioblastoma (glioblastoma multiforme, GBM).
MATERIALS/METHODS
This study was performed through in vitro experiments using C6 cells. The toxicity dependence of DHA and transferrin (TF) on time and concentration was analyzed by MTT assay and cell cycle assay. Observations of cellular morphology were recorded with an optical microscope and color digital camera. The anti-cancer mechanism of DHA and TF against GBM were studied by flow cytometry with Annexin V and caspase 3/7.
RESULTS
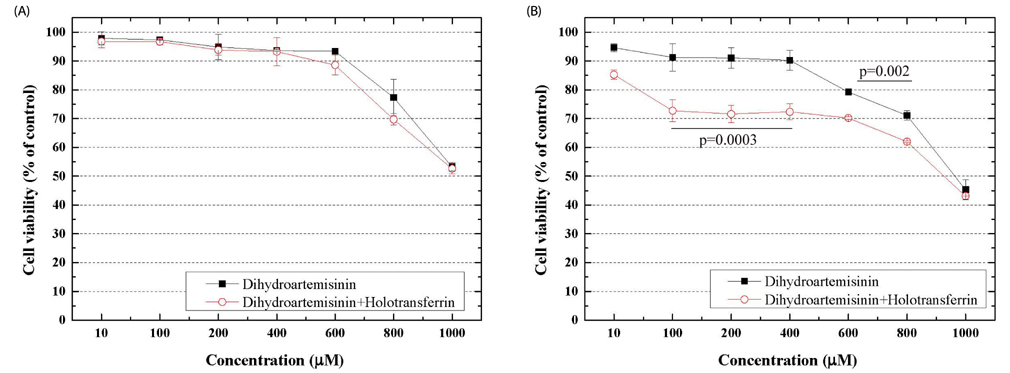
MTT assay revealed that TF enhanced the cytotoxicity of DHA against C6 cells. An Annexin V immune-precipitation assay showed that the percentages of apoptosis of cells treated with TF, DHA alone, DHA in combination with TF, and the control group were 7.15 ± 4.15%, 34.3 ± 5.15%, 66.42 ± 5.98%, and 1.2 ± 0.15%, respectively. The results of the Annexin V assay were consistent with those of the MTT assay. DHA induced apoptosis in C6 cells through DNA damage, and TF enhanced the effects of DHA.
CONCLUSION
The results of this study demonstrated that DHA, the derivative of the active ingredient in Gaeddongssuk, is effective against GBM, apparently via inhibition of cancer cell proliferation by a pharmacological effect. The role of transferrin as an allosteric activator in the GBM therapeutic efficacy of DHA was also confirmed.
MeSH Terms
Figure
Reference
-
1. Huang L, Liu JF, Liu LX, Li DF, Zhang Y, Nui HZ, Song HY, Zhang CY. Antipyretic and anti-inflammatory effects of Artemisia annua L. Zhongguo Zhong Yao Za Zhi. 1993; 18:44–48. 63–64.2. Juteau F, Masotti V, Bessière JM, Dherbomez M, Viano J. Antibacterial and antioxidant activities of Artemisia annua essential oil. Fitoterapia. 2002; 73:532–535.
Article3. Habib M, Waheed I. Evaluation of anti-nociceptive, anti-inflammatory and antipyretic activities of Artemisia scoparia hydromethanolic extract. J Ethnopharmacol. 2013; 145:18–24.
Article4. Sen R, Bandyopadhyay S, Dutta A, Mandal G, Ganguly S, Saha P, Chatterjee M. Artemisinin triggers induction of cell-cycle arrest and apoptosis in Leishmania donovani promastigotes. J Med Microbiol. 2007; 56:1213–1218.
Article5. Gao X, Luo Z, Xiang T, Wang K, Li J, Wang P. Dihydroartemisinin induces endoplasmic reticulum stress-mediated apoptosis in HepG2 human hepatoma cells. Tumori. 2011; 97:771–780.
Article6. Chaturvedi D, Goswami A, Saikia PP, Barua NC, Rao PG. Artemisinin and its derivatives: a novel class of anti-malarial and anti-cancer agents. Chem Soc Rev. 2010; 39:435–454.
Article7. Chertok B, Moffat BA, David AE, Yu F, Bergemann C, Ross BD, Yang VC. Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors. Biomaterials. 2008; 29:487–496.
Article8. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352:987–996.
Article9. Kim SJ, Kim MS, Lee JW, Lee CH, Yoo H, Shin SH, Park MJ, Lee SH. Dihydroartemisinin enhances radiosensitivity of human glioma cells in vitro. J Cancer Res Clin Oncol. 2006; 132:129–135.
Article10. Chen H, Sun B, Pan S, Jiang H, Sun X. Dihydroartemisinin inhibits growth of pancreatic cancer cells in vitro and in vivo. Anticancer Drugs. 2009; 20:131–140.
Article11. Wu ZP, Gao CW, Wu YG, Zhu QS, Yan Chen, Xin Liu, Chuen Liu. Inhibitive effect of artemether on tumor growth and angiogenesis in the rat C6 orthotopic brain gliomas model. Integr Cancer Ther. 2009; 8:88–92.
Article12. Lai H, Singh NP. Selective cancer cell cytotoxicity from exposure to dihydroartemisinin and holotransferrin. Cancer Lett. 1995; 91:41–46.
Article13. Singh NP, Lai HC. Artemisinin induces apoptosis in human cancer cells. Anticancer Res. 2004; 24:2277–2280.14. van Agtmael MA, Eggelte TA, van Boxtel CJ. Artemisinin drugs in the treatment of malaria: from medicinal herb to registered medication. Trends Pharmacol Sci. 1999; 20:199–205.
Article15. Tan W, Lu J, Huang M, Li Y, Chen M, Wu G, Gong J, Zhong Z, Xu Z, Dang Y, Guo J, Chen X, Wang Y. Anti-cancer natural products isolated from Chinese medicinal herbs. Chin Med. 2011; 6:27–42.
Article16. Chen HH, Zhou HJ, Fang X. Inhibition of human cancer cell line growth and human umbilical vein endothelial cell angiogenesis by artemisinin derivatives in vitro. Pharmacol Res. 2003; 48:231–236.
Article17. Singh NP, Lai H. Selective toxicity of dihydroartemisinin and holotransferrin toward human breast cancer cells. Life Sci. 2001; 70:49–56.
Article18. Singh NP, Lai HC. Synergistic cytotoxicity of artemisinin and sodium butyrate on human cancer cells. Anticancer Res. 2005; 25 6B:4325–4331.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Postoperative Radiation Therapy of Astrocytoma and Glioblastoma Multiforme
- Antimalarial activity of thiophenyl- and benzenesulfonyl-dihydroartemisinin
- Transferrin Receptors in Gliomas and its Relationship with Flow Cytometric Analysis
- Radiotherapy Results of Malignant Astrocytoma and Glioblastoma Multiforme
- Radiotherapy Results of Brain Astrocytoma and glioblastoma Multiforme