Cancer Res Treat.
2017 Jul;49(3):816-823. 10.4143/crt.2016.417.
A Randomized Phase II Study of Leucovorin/5-Fluorouracil with or without Oxaliplatin (LV5FU2 vs. FOLFOX) for Curatively-Resected, Node-Positive Esophageal Squamous Cell Carcinoma
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Internal Medicine, Dongtan Sacred Heart Hospital, Hallym University of College of Medicine, Hwaseong, Korea.
- 2Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. tntntn3@gmail.com
- 3Department of Thoracic and Cardiovascular Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2388327
- DOI: http://doi.org/10.4143/crt.2016.417
Abstract
- PURPOSE
The optimal perioperative treatment for resectable esophageal squamous cell carcinoma (ESCC) remains controversial. We evaluated the efficacy and safety of leucovorin and 5-fluorouracil (LV5FU2) and LV5FU2 plus oxaliplatin (FOLFOX) combination chemotherapies administered adjuvantly for curatively-resected, node-positive ESCC.
MATERIALS AND METHODS
Patients with pathologically node-positive esophageal cancer after curative R0 resection were enrolled and randomly assigned to receive LV5FU2 or FOLFOX biweekly for up to eight cycles. The primary endpoint was disease-free survival (DFS).
RESULTS
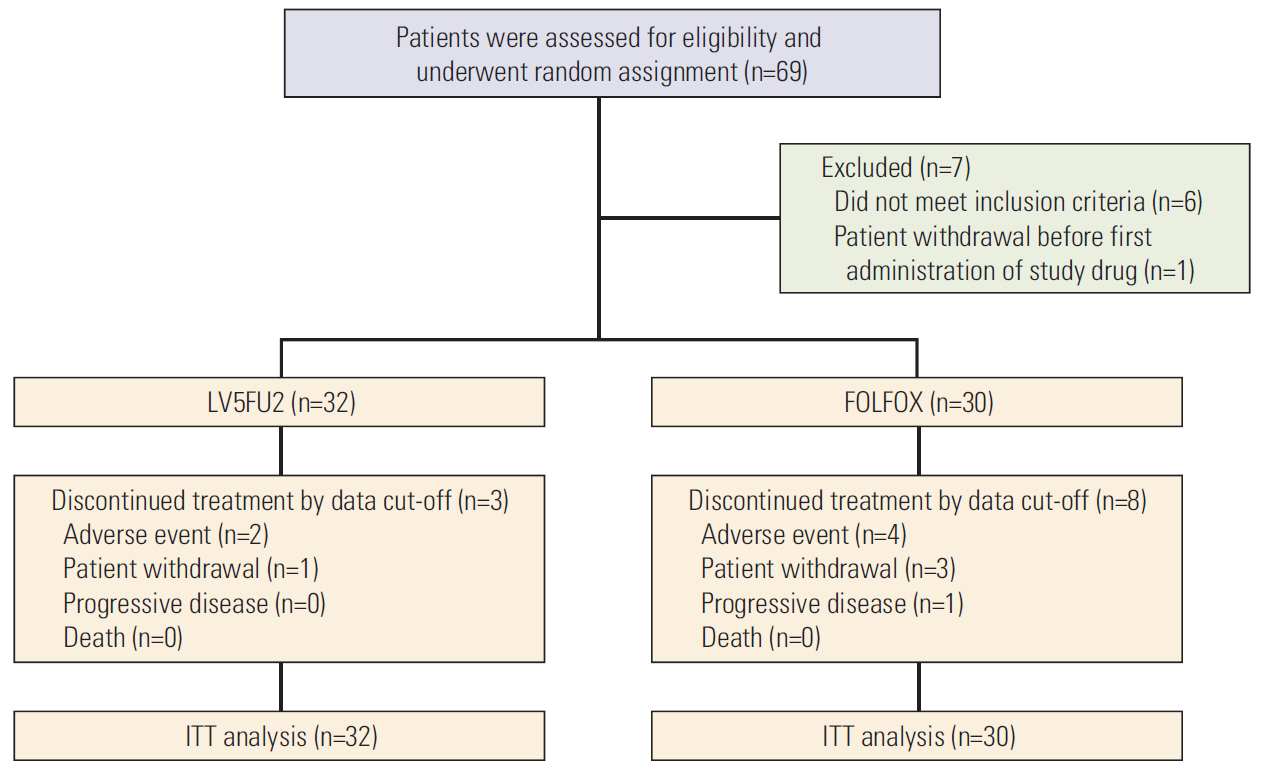
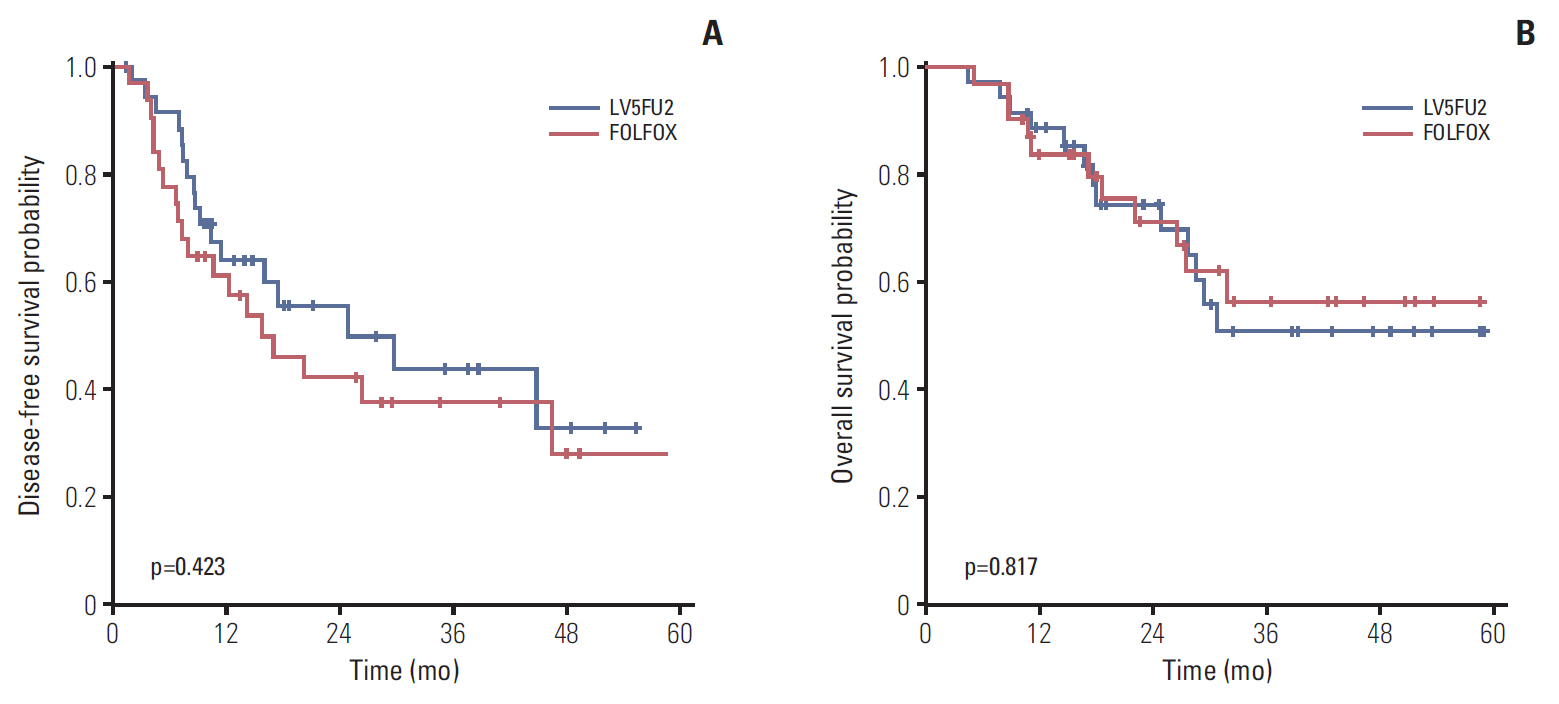
Between 2011 and 2015, 62 patients were randomized into the two treatment groups (32 in the LV5FU2 arm and 30 in the FOLFOX arm). The median age was 60 years and both groups had similar pathologic characteristics in tumor, nodal status, and location. Treatment completion rates were similarly high in both groups. The DFS rate at 12 months was 67% in the LV5FU2 group and 63% in the FOLFOX group with a hazard ratio of 1.3 (95% confidence interval [CI], 0.66 to 2.62). After a median follow-up period of 27 months, the median DFS was 29.6 months (95% CI, 4.9 to 54.2) in the LV5FU2 arm and 16.8 months (95% CI, 7.5 to 26.1) in the FOLFOX arm (p=0.428), respectively, while the median overall survival was not reached in either arm. Grade 3 or 4 neutropenia was more frequent in patients in the FOLFOX arm than the LV5FU2 arm (20.0% vs. 3.1%).
CONCLUSION
The addition of oxaliplatin (FOLFOX) did not lead to better efficacy compared to LV5FU2 chemotherapy in an adjuvant setting in node-positive ESCC patients.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
Article2. Oh CM, Won YJ, Jung KW, Kong HJ, Cho H, Lee JK, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2013. Cancer Res Treat. 2016; 48:436–50.
Article3. Layke JC, Lopez PP. Esophageal cancer: a review and update. Am Fam Physician. 2006; 73:2187–94.4. Steup WH, De Leyn P, Deneffe G, Van Raemdonck D, Coosemans W, Lerut T. Tumors of the esophagogastric junction: long-term survival in relation to the pattern of lymph node metastasis and a critical analysis of the accuracy or inaccuracy of pTNM classification. J Thorac Cardiovasc Surg. 1996; 111:85–94.5. Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma:an updated meta-analysis. Lancet Oncol. 2011; 12:681–92.6. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012; 366:2074–84.
Article7. Shapiro J, van Lanschot JJ, Hulshof MC, van Hagen P, van Berge Henegouwen MI, Wijnhoven BP, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015; 16:1090–8.
Article8. Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009; 27:5062–7.
Article9. Yano M, Motoori M, Tanaka K, Kishi K, Fujiwara Y, Shingai T, et al. Preoperative staging of clinically node-negative esophageal cancer by the combination of 18F-fluorodeoxyglucose positron emission tomography and computed tomography (FDG–PET/CT). Esophagus. 2012; 9:210–6.
Article10. Ando N, Iizuka T, Ide H, Ishida K, Shinoda M, Nishimaki T, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study: JCOG-9204. J Clin Oncol. 2003; 21:4592–6.11. Bleiberg H, Conroy T, Paillot B, Lacave AJ, Blijham G, Jacob JH, et al. Randomised phase II study of cisplatin and 5-fluorouracil (5-FU) versus cisplatin alone in advanced squamous cell oesophageal cancer. Eur J Cancer. 1997; 33:1216–20.
Article12. Shiozaki A, Yamagishi H, Itoi H, Fujiwara H, Kikuchi S, Okamoto K, et al. Long-term administration of low-dose cisplatin plus 5-fluorouracil prolongs the postoperative survival of patients with esophageal cancer. Oncol Rep. 2005; 13:667–72.
Article13. Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012; 19:68–74.
Article14. Pasquali S, Yim G, Vohra RS, Mocellin S, Nyanhongo D, Marriott P, et al. Survival after neoadjuvant and adjuvant treatments compared to surgery alone for resectable esophageal carcinoma: a network meta-analysis. Ann Surg. 2017; 265:481–91.15. Conroy T, Galais MP, Raoul JL, Bouche O, Gourgou-Bourgade S, Douillard JY, et al. Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): final results of a randomised, phase 2/3 trial. Lancet Oncol. 2014; 15:305–14.
Article16. Mauer AM, Kraut EH, Krauss SA, Ansari RH, Kasza K, Szeto L, et al. Phase II trial of oxaliplatin, leucovorin and fluorouracil in patients with advanced carcinoma of the esophagus. Ann Oncol. 2005; 16:1320–5.
Article17. Hsu PK, Huang CS, Wang BY, Wu YC, Hsu WH. Survival benefits of postoperative chemoradiation for lymph node-positive esophageal squamous cell carcinoma. Ann Thorac Surg. 2014; 97:1734–41.
Article18. Lee J, Lee KE, Im YH, Kang WK, Park K, Kim K, et al. Adjuvant chemotherapy with 5-fluorouracil and cisplatin in lymph node-positive thoracic esophageal squamous cell carcinoma. Ann Thorac Surg. 2005; 80:1170–5.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Interstitial Lung Disease Associated with Combination Chemotherapy of Oxaliplatin, 5-Fluorouracil, and Leucovorin
- Oxaliplatin-induced Pulmonary Fibrosis: Two Case Reports
- Leucovorin-induced Hypersensitivity Reaction in a Patient with Metastatic Colorectal Cancer Treated with Cetuximab Plus FOLFOX Chemotherapy: A Case Report
- Diffuse alveolar damage during chemotherapy with oxaliplatin, 5-fluorouracil and leucovorin
- Pulmonary Fibrosis Under Chemotherapy with Oxaliplatin, 5-fluorouracil, and Leucovorin