Cancer Res Treat.
2017 Jul;49(3):807-815. 10.4143/crt.2016.326.
CA19-9 or CEA Decline after the First Cycle of Treatment Predicts Survival in Advanced Biliary Tract Cancer Patients Treated with S-1 and Cisplatin Chemotherapy
- Affiliations
-
- 1Department of Internal Medicine and Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea. moisa@snu.ac.kr
- 2Translational Medicine Major, Department of Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 4Department of Internal Medicine, Jeju National University Hospital, Jeju, Korea.
- 5Department of Internal Medicine, Korea Cancer Center Hospital, Seoul, Korea.
- 6Department of Internal Medicine, Seoul Municipal Boramae Hospital, Seoul, Korea.
- KMID: 2388326
- DOI: http://doi.org/10.4143/crt.2016.326
Abstract
- PURPOSE
While tumor markers (carbohydrate antigen 19-9 [CA 19-9] and carcinoembryonic antigen [CEA]) can aid in the diagnosis of biliary tract cancer, their prognostic role has not been clearly elucidated. Therefore, this study was conducted to evaluate the prognostic role of tumor markers and tumor marker change in patients with advanced biliary tract cancer.
MATERIALS AND METHODS
Patients with pathologically proven metastatic or relapsed biliary tract cancer who were treated in a phase II trial of first-line S-1 and cisplatin chemotherapy were enrolled. Serum tumor markers were measured at baseline and after the first cycle of chemotherapy.
RESULTS
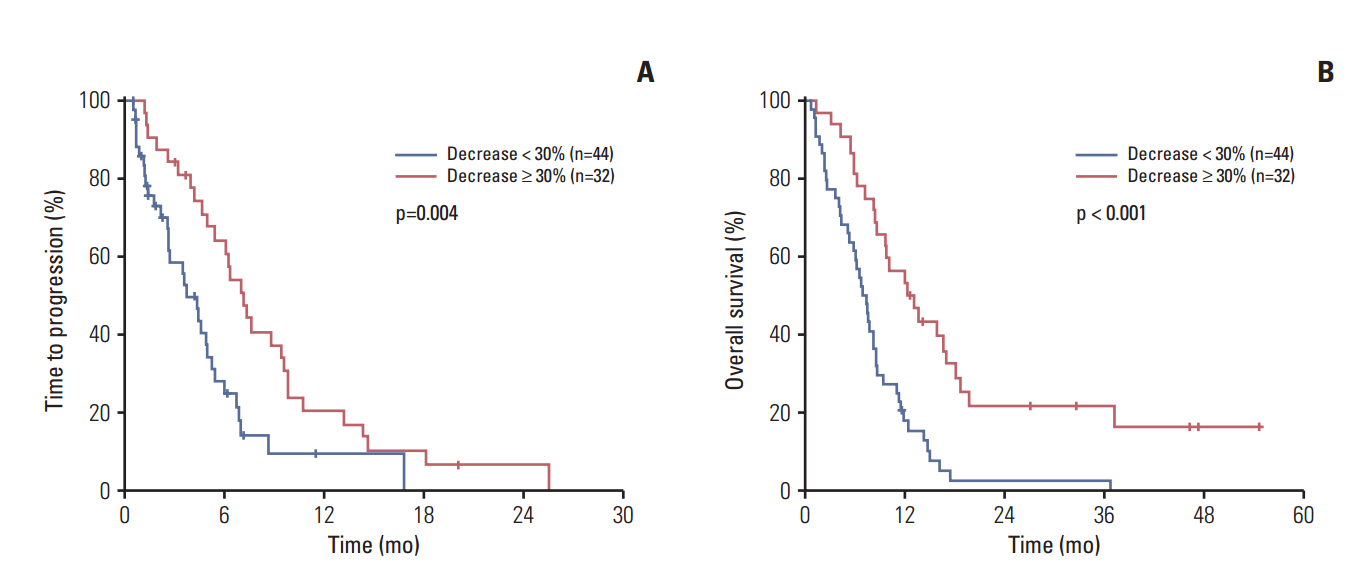
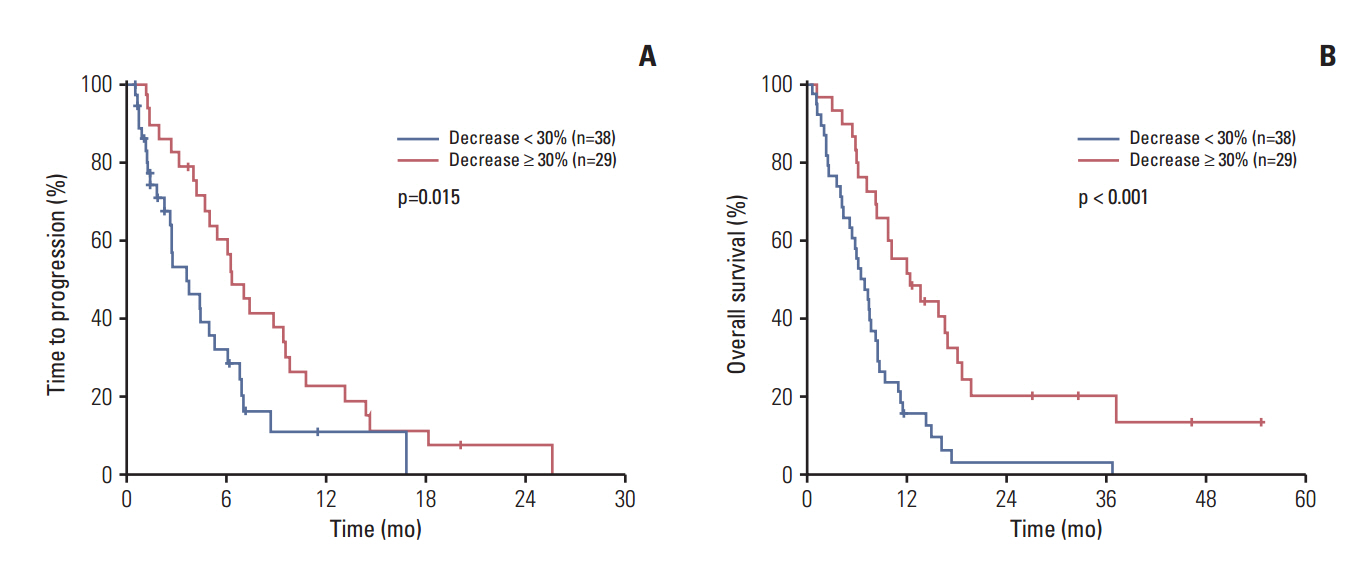
Among a total of 104 patients, 80 (77%) had elevated baseline tumor markers (69 with CA 19-9 elevation and 40 with CEA). A decline ≥ 30% of the elevated tumor marker level after the first cycle of chemotherapy conferred an improved time to progression (TTP), overall survival (OS), and better chemotherapy response. Multivariate analysis revealed tumor marker decline as an independent positive prognostic factor of TTP (adjusted hazard ratio [HR], 0.44; p=0.003) and OS (adjusted HR, 0.37; p < 0.001). Subgroup analysis revealed similar results in each group of patients with CA 19-9 elevation and CEA elevation. In addition, elevated baseline CEA was associated with poor survival in both univariate and multivariate analysis.
CONCLUSION
Tumor marker decline was associated with improved survival in biliary tract cancer. Measuring tumor marker after the first cycle of chemotherapy can be used as an early assessment of treatment outcome.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013; 63:11–30.
Article2. Randi G, Malvezzi M, Levi F, Ferlay J, Negri E, Franceschi S, et al. Epidemiology of biliary tract cancers: an update. Ann Oncol. 2009; 20:146–59.
Article3. Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz J, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001; 234:507–17.
Article4. Glimelius B, Hoffman K, Sjoden PO, Jacobsson G, Sellstrom H, Enander LK, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol. 1996; 7:593–600.
Article5. Sharma A, Dwary AD, Mohanti BK, Deo SV, Pal S, Sreenivas V, et al. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. J Clin Oncol. 2010; 28:4581–6.
Article6. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010; 362:1273–81.
Article7. Park JO, Oh DY, Hsu C, Chen JS, Chen LT, Orlando M, et al. Gemcitabine plus cisplatin for advanced biliary tract cancer: a systematic review. Cancer Res Treat. 2015; 47:343–61.
Article8. Kang MJ, Lee JL, Kim TW, Lee SS, Ahn S, Park DH, et al. Randomized phase II trial of S-1 and cisplatin versus gemcitabine and cisplatin in patients with advanced biliary tract adenocarcinoma. Acta Oncol. 2012; 51:860–6.
Article9. Kornek GV, Schuell B, Laengle F, Gruenberger T, Penz M, Karall K, et al. Mitomycin C in combination with capecitabine or biweekly high-dose gemcitabine in patients with advanced biliary tract cancer: a randomised phase II trial. Ann Oncol. 2004; 15:478–83.10. Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer. 2007; 96:896–902.
Article11. Lee J, Hong TH, Lee IS, You YK, Lee MA. Comparison of the efficacy between gemcitabine-cisplatin and capecitabine-cisplatin combination chemotherapy for advanced biliary tract cancer. Cancer Res Treat. 2015; 47:259–65.
Article12. Sasaki T, Isayama H, Nakai Y, Koike K. Current status of chemotherapy for the treatment of advanced biliary tract cancer. Korean J Intern Med. 2013; 28:515–24.
Article13. Park I, Lee JL, Ryu MH, Kim TW, Lee SS, Park DH, et al. Prognostic factors and predictive model in patients with advanced biliary tract adenocarcinoma receiving first-line palliative chemotherapy. Cancer. 2009; 115:4148–55.
Article14. Harder J, Kummer O, Olschewski M, Otto F, Blum HE, Opitz O. Prognostic relevance of carbohydrate antigen 19-9 levels in patients with advanced biliary tract cancer. Cancer Epidemiol Biomarkers Prev. 2007; 16:2097–100.
Article15. Grunnet M, Christensen IJ, Lassen U, Jensen LH, Lydolph M, Knox JJ, et al. Decline in CA19-9 during chemotherapy predicts survival in four independent cohorts of patients with inoperable bile duct cancer. Eur J Cancer. 2015; 51:1381–8.
Article16. Kim YJ, Im SA, Kim HG, Oh SY, Lee KW, Choi IS, et al. A phase II trial of S-1 and cisplatin in patients with metastatic or relapsed biliary tract cancer. Ann Oncol. 2008; 19:99–103.
Article17. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.
Article18. Duffy MJ. Carcinoembryonic antigen as a marker for colorectal cancer: is it clinically useful? Clin Chem. 2001; 47:624–30.
Article19. Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: an evidence based appraisal. J Gastrointest Oncol. 2012; 3:105–19.20. Sasaki T, Isayama H, Nakai Y, Togawa O, Kogure H, Ito Y, et al. Prognostic factors in patients with advanced biliary tract cancer receiving chemotherapy. Cancer Chemother Pharmacol. 2011; 67:847–53.
Article21. Siqueira E, Schoen RE, Silverman W, Martin J, Rabinovitz M, Weissfeld JL, et al. Detecting cholangiocarcinoma in patients with primary sclerosing cholangitis. Gastrointest Endosc. 2002; 56:40–7.
Article22. Ramage JK, Donaghy A, Farrant JM, Iorns R, Williams R. Serum tumor markers for the diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Gastroenterology. 1995; 108:865–9.
Article23. Levy C, Lymp J, Angulo P, Gores GJ, Larusso N, Lindor KD. The value of serum CA 19-9 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis. Dig Dis Sci. 2005; 50:1734–40.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current status of chemotherapy for the treatment of advanced biliary tract cancer
- Chemotherapy for Biliary Tract Cancer
- The Prognostic Significance of the Preoperative Serum CEA, CA19-9 and AFP Levels in Gastric Cancer Patients
- Novel Palliative Chemotherapy for Cholangiocarcinoma
- Effect of Biliary Drainage on the Diagnostic Values of Serum and Bile CEA and CA19-9 in Patient swith Obstructive Jaundice and/or Acute Cholangitis