Cancer Res Treat.
2017 Jul;49(3):717-726. 10.4143/crt.2016.271.
Outcomes of Treatment for Malignant Peripheral Nerve Sheath Tumors: Different Clinical Features Associated with Neurofibromatosis Type 1
- Affiliations
-
- 1Division of Pediatric Hemato-oncology, Department of Pediatrics, Yonsei University Health System, Yonsei University College of Medicine, Seoul, Korea. jwhan@yuhs.ac
- 2Department of Pediatric Hematology and Oncology, Yonsei Cancer Center, Yonsei University Health System, Seoul, Korea.
- 3Department of Pathology, Yonsei University Health System, Yonsei University College of Medicine, Seoul, Korea.
- 4Division of Medical Oncology, Department of Internal Medicine, Yonsei University Health System, Yonsei University College of Medicine, Seoul, Korea.
- 5Department of Orthopedic Surgery, Yonsei University Health System, Yonsei University College of Medicine, Seoul, Korea.
- 6Department of Radiation Oncology, Yonsei University Health System, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2388317
- DOI: http://doi.org/10.4143/crt.2016.271
Abstract
- PURPOSE
Malignant peripheral nerve sheath tumors (MPNSTs) are a rare subtype of sarcoma that occur spontaneously or in association with neurofibromatosis type 1 (NF-1). This study aimed to clinically differentiate these types of MPNSTs.
MATERIALS AND METHODS
The study reviewed 95 patients diagnosed with and treated for MPNST at Yonsei University Health System, Seoul, Korea over a 27-year period. The clinical characteristics, prognostic factors, and treatment outcomes of sporadic MPNST (sMPNST) and NF-1 associated MPNST (NF-MPNST) cases were compared.
RESULTS
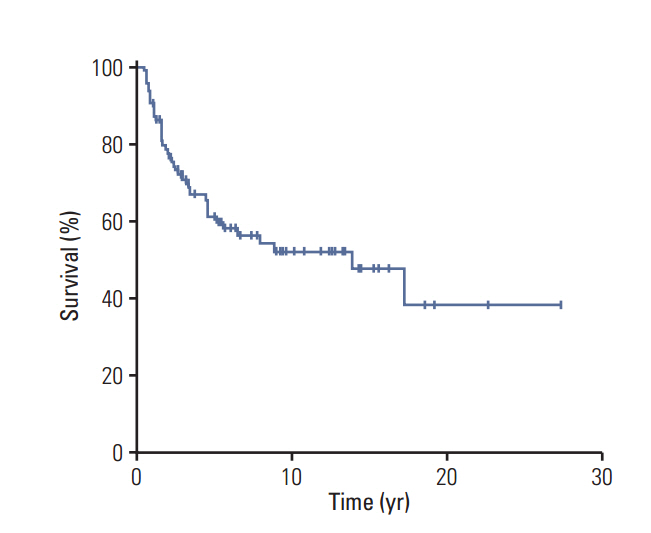
Patients with NF-MPNST had a significantly lower median age (32 years vs. 45 years for sMPNST, p=0.012), significantly larger median tumor size (8.2 cm vs. 5.0 cm for sMPNST, p < 0.001), and significantly larger numbers of imaging studies and surgeries (p=0.004 and p < 0.001, respectively). The 10-year overall survival (OS) rate of the patients with MPNST was 52±6%. Among the patients with localized MPNST, patients with NF-MPNST had a significantly lower 10-year OS rate (45±11% vs. 60±8% for sMPNST, p=0.046). Univariate analysis revealed the resection margin, pathology grade, and metastasis to be significant factors affecting the OS (p=0.001, p=0.020, and p < 0.001, respectively). Multivariate analysis of the patients with localized MPNST identified R2 resection and G1 as significant prognostic factors for OS.
CONCLUSION
NF-MPNST has different clinical features from sMPNST and requires more careful management. Further study will be needed to develop specific management plans for NF-MPNST.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Superficial malignant peripheral nerve sheath tumor from recurrent neurofibroma in the abdominal wall of a patient without neurofibromatosis type 1
Chang Yeon Jung, Jung Min Bae, Joon Hyuk Choi, Ki Hoon Jung
Yeungnam Univ J Med. 2018;36(1):63-66. doi: 10.12701/yujm.2019.00031.
Reference
-
References
1. Amirian ES, Goodman JC, New P, Scheurer ME. Pediatric and adult malignant peripheral nerve sheath tumors: an analysis of data from the surveillance, epidemiology, and end results program. J Neurooncol. 2014; 116:609–16.
Article2. Tucker T, Wolkenstein P, Revuz J, Zeller J, Friedman JM. Association between benign and malignant peripheral nerve sheath tumors in NF1. Neurology. 2005; 65:205–11.
Article3. Ferner RE, Gutmann DH. International consensus statement on malignant peripheral nerve sheath tumors in neurofibromatosis. Cancer Res. 2002; 62:1573–7.4. Baehring JM, Betensky RA, Batchelor TT. Malignant peripheral nerve sheath tumor: the clinical spectrum and outcome of treatment. Neurology. 2003; 61:696–8.5. Carli M, Ferrari A, Mattke A, Zanetti I, Casanova M, Bisogno G, et al. Pediatric malignant peripheral nerve sheath tumor: the Italian and German soft tissue sarcoma cooperative group. J Clin Oncol. 2005; 23:8422–30.
Article6. Stucky CC, Johnson KN, Gray RJ, Pockaj BA, Ocal IT, Rose PS, et al. Malignant peripheral nerve sheath tumors (MPNST): the Mayo Clinic experience. Ann Surg Oncol. 2012; 19:878–85.
Article7. LaFemina J, Qin LX, Moraco NH, Antonescu CR, Fields RC, Crago AM, et al. Oncologic outcomes of sporadic, neurofibromatosis-associated, and radiation-induced malignant peripheral nerve sheath tumors. Ann Surg Oncol. 2013; 20:66–72.
Article8. Hirbe AC, Gutmann DH. Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol. 2014; 13:834–43.
Article9. DeBella K, Szudek J, Friedman JM. Use of the national institutes of health criteria for diagnosis of neurofibromatosis 1 in children. Pediatrics. 2000; 105(3 Pt 1):608–14.
Article10. Gutmann DH, McLellan MD, Hussain I, Wallis JW, Fulton LL, Fulton RS, et al. Somatic neurofibromatosis type 1 (NF1) inactivation characterizes NF1-associated pilocytic astrocytoma. Genome Res. 2013; 23:431–9.
Article11. Porter DE, Prasad V, Foster L, Dall GF, Birch R, Grimer RJ. Survival in malignant peripheral nerve sheath tumours: a comparison between sporadic and neurofibromatosis type 1-associated tumours. Sarcoma. 2009; 2009:756395.
Article12. Kolberg M, Holand M, Agesen TH, Brekke HR, Liestol K, Hall KS, et al. Survival meta-analyses for >1800 malignant peripheral nerve sheath tumor patients with and without neurofibromatosis type 1. Neuro Oncol. 2013; 15:135–47.13. Cashen DV, Parisien RC, Raskin K, Hornicek FJ, Gebhardt MC, Mankin HJ. Survival data for patients with malignant schwannoma. Clin Orthop Relat Res. 2004; (426):69–73.
Article14. Yu J, Deshmukh H, Payton JE, Dunham C, Scheithauer BW, Tihan T, et al. Array-based comparative genomic hybridization identifies CDK4 and FOXM1 alterations as independent predictors of survival in malignant peripheral nerve sheath tumor. Clin Cancer Res. 2011; 17:1924–34.
Article15. Anghileri M, Miceli R, Fiore M, Mariani L, Ferrari A, Mussi C, et al. Malignant peripheral nerve sheath tumors: prognostic factors and survival in a series of patients treated at a single institution. Cancer. 2006; 107:1065–74.16. Zou C, Smith KD, Liu J, Lahat G, Myers S, Wang WL, et al. Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Ann Surg. 2009; 249:1014–22.
Article17. Deyrup AT, Weiss SW. Grading of soft tissue sarcomas: the challenge of providing precise information in an imprecise world. Histopathology. 2006; 48:42–50.
Article18. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17:1471–4.
Article19. Zehou O, Fabre E, Zelek L, Sbidian E, Ortonne N, Banu E, et al. Chemotherapy for the treatment of malignant peripheral nerve sheath tumors in neurofibromatosis 1: a 10-year institutional review. Orphanet J Rare Dis. 2013; 8:127.
Article20. Evans DG, Baser ME, McGaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet. 2002; 39:311–4.
Article21. deCou JM, Rao BN, Parham DM, Lobe TE, Bowman L, Pappo AS, et al. Malignant peripheral nerve sheath tumors: the St. Jude Children's Research Hospital experience. Ann Surg Oncol. 1995; 2:524–9.
Article22. Hegedus B, Banerjee D, Yeh TH, Rothermich S, Perry A, Rubin JB, et al. Preclinical cancer therapy in a mouse model of neurofibromatosis-1 optic glioma. Cancer Res. 2008; 68:1520–8.
Article23. Kahn J, Gillespie A, Tsokos M, Ondos J, Dombi E, Camphausen K, et al. Radiation therapy in management of sporadic and neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors. Front Oncol. 2014; 4:324.
Article24. Sordillo PP, Helson L, Hajdu SI, Magill GB, Kosloff C, Golbey RB, et al. Malignant schwannoma: clinical characteristics, survival, and response to therapy. Cancer. 1981; 47:2503–9.25. Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer. 1986; 57:2006–21.
Article26. Korf BR. Malignancy in neurofibromatosis type 1. Oncologist. 2000; 5:477–85.
Article27. Meehan RS, Chen AP. New treatment option for ovarian cancer: PARP inhibitors. Gynecol Oncol Res Pract. 2016; 3:3.
Article28. Curatolo P, Moavero R, de Vries PJ. Neurological and neuropsychiatric aspects of tuberous sclerosis complex. Lancet Neurol. 2015; 14:733–45.
Article29. Colman SD, Williams CA, Wallace MR. Benign neurofibromas in type 1 neurofibromatosis (NF1) show somatic deletions of the NF1 gene. Nat Genet. 1995; 11:90–2.
Article30. Carroll SL. The challenge of cancer genomics in rare nervous system neoplasms: malignant peripheral nerve sheath tumors as a paradigm for cross-species comparative oncogenomics. Am J Pathol. 2016; 186:464–77.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Malignant Peripheral Nerve Sheath Tumor of the Cauda Equina in Type I Neurofibromatosis: Case Report
- Malignant Peripheral Nerve Sheath Tumor of the Larynx
- Concurrence of Malignant Peripheral Nerve Sheath Tumor at the Site of Complex Regional Pain Syndrome Type 1: A Case Report
- An intrathoracic malignant peripheral nerve sheath tumor in a neurofibromatosis type 1 patient
- A Case of Malignant Peripheral Nerve Sheath Tumor of the Neck Associated with Neurofibromatosis Type I