Cancer Res Treat.
2017 Jul;49(3):695-705. 10.4143/crt.2016.317.
Clinicopathologic Characteristics and Prognosis of Tongue Squamous Cell Carcinoma in Patients with and without a History of Radiation for Nasopharyngeal Carcinoma: A Matched Case-Control Study
- Affiliations
-
- 1Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China. ximian@sysucc.org.cn
- 2Department of Radiation Oncology, Sun Yat-sen University Cancer Center, Guangzhou, China.
- 3Department of Pathology, Sun Yat-sen University Cancer Center, Guangzhou, China.
- 4Department of Imaging Diagnosis and Interventional Center, Sun Yat-sen University Cancer Center, Guangzhou, China.
- 5Department of Medical Statistics and Epidemiology, School of Public Health, Sun Yat-sen University, Guangzhou, China.
- KMID: 2388315
- DOI: http://doi.org/10.4143/crt.2016.317
Abstract
- PURPOSE
Previous studies reported an association between an increased risk of tongue cancer and radiation treatment for nasopharyngeal carcinoma (NPC). This study compared the clinicopathologic characteristics and outcomes of tongue squamous cell carcinoma (TSCC) in patients with and without a history of radiotherapy for NPC.
MATERIALS AND METHODS
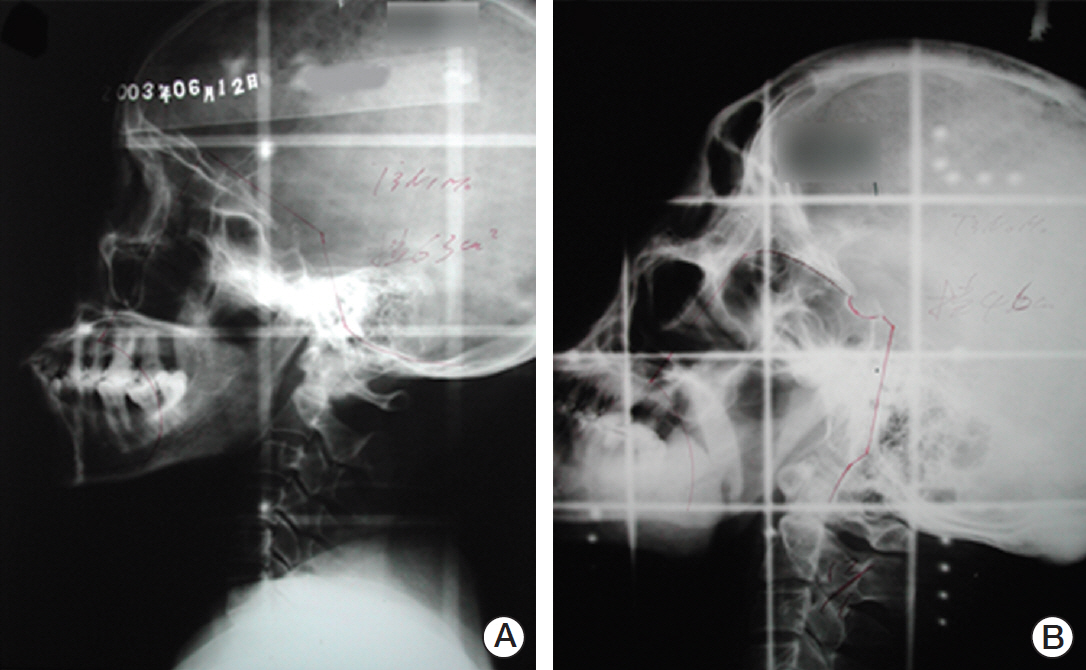
From 1965 to 2009, a total of 73 patients were diagnosed with TSCC with a history of radiotherapy for NPC. The patients were matched in a 1:3 ratio with patients with sporadic TSCC according to age, sex, and year of the TSCC diagnosis. The primary endpoint was the overall survival.
RESULTS
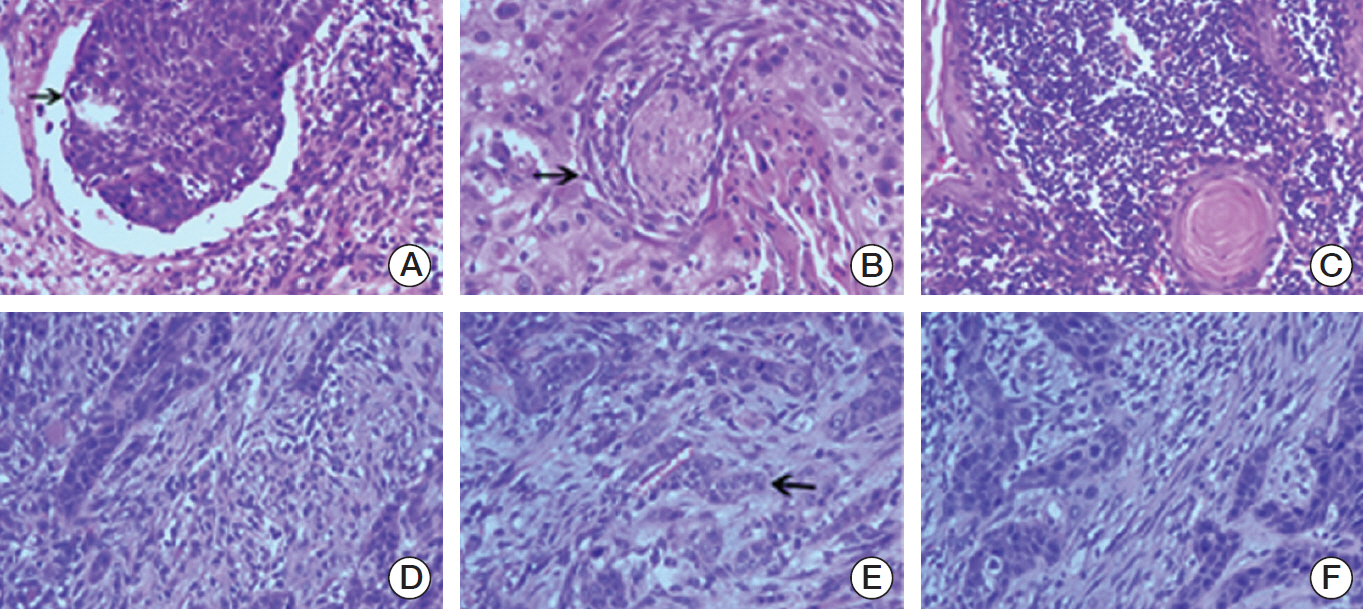
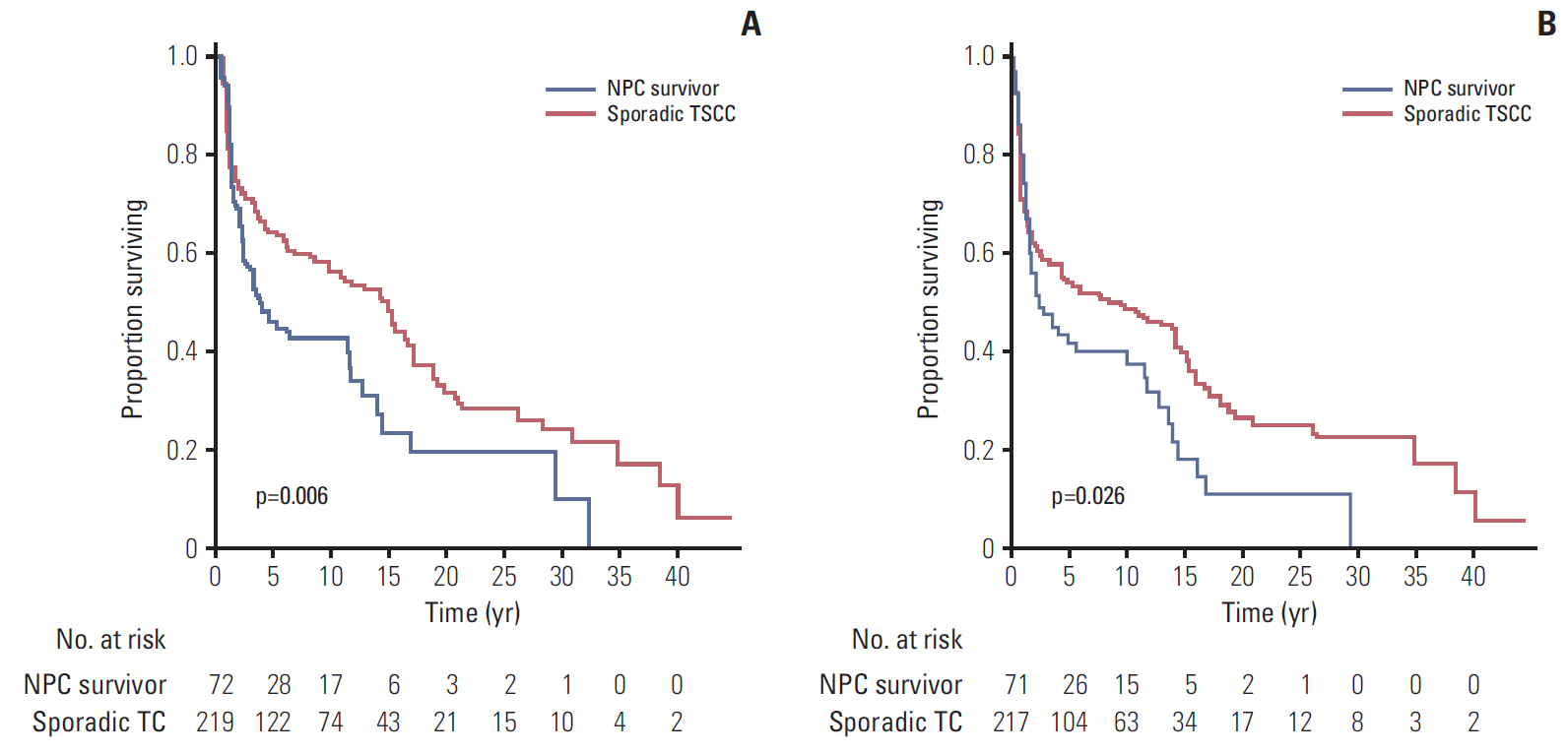
The median interval from NPC to TSCC was 82 months. The NPC survivors were more likely to be diagnosed with a more advanced T classification, less likely to have lymph node involvement, and more likely to have the tumor located in the dorsum of the tongue than sporadic TSCC. Regarding the histologic characteristics, the NPC survivors were more likely to have a weak lymphocytic host response, low tumor budding, and low risk of a worse pattern of invasion. The sporadic TSCC patients had a better overall survival (hazard ratio, 0.690; p=0.033) than the NPC survivors. In competing risks analysis, the cumulative incidence functions for the competing event (documented non-tongue cancer death) were significantly higher in the NPC survivors (Gray's test, p=0.001).
CONCLUSION
TSCC patients with a history of radiotherapy for NPC appear to have particular clinicopathologic features, a poorer survival, and are more likely to die from non-tongue cancer causes than those with sporadic TSCC.
MeSH Terms
Figure
Cited by 1 articles
-
Secondary Squamous Cell Carcinoma of the Oral Cavity after Nasopharyngeal Carcinoma
Liyuan Dai, Qigen Fang, Peng Li, Junfu Wu, Xu Zhang
Cancer Res Treat. 2020;52(1):109-116. doi: 10.4143/crt.2019.202.
Reference
-
References
1. Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet. 2005; 365:2041–54.
Article2. Lee AW, Sze WM, Au JS, Leung SF, Leung TW, Chua DT, et al. Treatment results for nasopharyngeal carcinoma in the modern era: the Hong Kong experience. Int J Radiat Oncol Biol Phys. 2005; 61:1107–16.
Article3. Tham IW, Hee SW, Yeo RM, Salleh PB, Lee J, Tan TW, et al. Treatment of nasopharyngeal carcinoma using intensity-modulated radiotherapy-the national cancer centre singapore experience. Int J Radiat Oncol Biol Phys. 2009; 75:1481–6.
Article4. Travis LB, Demark Wahnefried W, Allan JM, Wood ME, Ng AK. Aetiology, genetics and prevention of secondary neoplasms in adult cancer survivors. Nat Rev Clin Oncol. 2013; 10:289–301.
Article5. Chen MC, Feng IJ, Lu CH, Chen CC, Lin JT, Huang SH, et al. The incidence and risk of second primary cancers in patients with nasopharyngeal carcinoma: a population-based study in Taiwan over a 25-year period (1979-2003). Ann Oncol. 2008; 19:1180–6.
Article6. Scelo G, Boffetta P, Corbex M, Chia KS, Hemminki K, Friis S, et al. Second primary cancers in patients with nasopharyngeal carcinoma: a pooled analysis of 13 cancer registries. Cancer Causes Control. 2007; 18:269–78.
Article7. Kong L, Lu JJ, Hu C, Guo X, Wu Y, Zhang Y. The risk of second primary tumors in patients with nasopharyngeal carcinoma after definitive radiotherapy. Cancer. 2006; 107:1287–93.
Article8. Teo PM, Chan AT, Leung SF, Chau RM, Yu PK, King WW, et al. Increased incidence of tongue cancer after primary radiotherapy for nasopharyngeal carcinoma: the possibility of radiation carcinogenesis. Eur J Cancer. 1999; 35:219–25.9. Xi M, Liu SL, Zhao L, Shen JX, Zhang L, Zhang P, et al. Prognostic factors and survival in patients with radiation-related second malignant neoplasms following radiotherapy for nasopharyngeal carcinoma. PLoS One. 2013; 8:e84586.
Article10. Cahan WG, Woodard HQ, Higinbotham NL, Stewart FW, Coley BL. Sarcoma arising in irradiated bone: report of eleven cases: 1948. Cancer. 1998; 82:8–34.11. Arlen M, Higinbotham NL, Huvos AG, Marcove RC, Miller T, Shah IC. Radiation-induced sarcoma of bone. Cancer. 1971; 28:1087–99.
Article12. Min H, Hong M, Ma J, Zhang E, Zheng Q, Zhang J, et al. A new staging system for nasopharyngeal carcinoma in China. Int J Radiat Oncol Biol Phys. 1994; 30:1037–42.13. Tao CJ, Liu X, Tang LL, Mao YP, Chen L, Li WF, et al. Prognostic scoring system for locoregional control among the patients with nasopharyngeal carcinoma treated by intensity-modulated radiotherapy. Chin J Cancer. 2013; 32:494–501.
Article14. Maleki S, Schlecht NF, Keller C, Diaz J, Moss J, Prystowsky MB, et al. Lymphocytic host response to oral squamous cell carcinoma: an adaptive T-cell response at the tumor interface. Head Neck Pathol. 2011; 5:117–22.
Article15. Almangush A, Bello IO, Keski-Santti H, Makinen LK, Kauppila JH, Pukkila M, et al. Depth of invasion, tumor budding, and worst pattern of invasion: prognostic indicators in early-stage oral tongue cancer. Head Neck. 2014; 36:811–8.
Article16. Ueno H, Price AB, Wilkinson KH, Jass JR, Mochizuki H, Talbot IC. A new prognostic staging system for rectal cancer. Ann Surg. 2004; 240:832–9.
Article17. Wang C, Huang H, Huang Z, Wang A, Chen X, Huang L, et al. Tumor budding correlates with poor prognosis and epithelial-mesenchymal transition in tongue squamous cell carcinoma. J Oral Pathol Med. 2011; 40:545–51.
Article18. Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988; 16:1141–54.
Article19. Wolden SL, Lamborn KR, Cleary SF, Tate DJ, Donaldson SS. Second cancers following pediatric Hodgkin's disease. J Clin Oncol. 1998; 16:536–44.
Article20. Qin WJ, Luo W, Lin SR, Sun Y, Li FM, Liu XQ, et al. Sparing normal oral tissues with individual dental stent in radiotherapy for primary nasopharyngeal carcinoma patients. Ai Zheng. 2007; 26:285–9.21. Brandwein-Gensler M, Smith RV, Wang B, Penner C, Theilken A, Broughel D, et al. Validation of the histologic risk model in a new cohort of patients with head and neck squamous cell carcinoma. Am J Surg Pathol. 2010; 34:676–88.
Article22. Xie N, Wang C, Liu X, Li R, Hou J, Chen X, et al. Tumor budding correlates with occult cervical lymph node metastasis and poor prognosis in clinical early-stage tongue squamous cell carcinoma. J Oral Pathol Med. 2015; 44:266–72.
Article23. Paleri V, Wight RG, Silver CE, Haigentz M Jr, Takes RP, Bradley PJ, et al. Comorbidity in head and neck cancer: a critical appraisal and recommendations for practice. Oral Oncol. 2010; 46:712–9.
Article24. Sanna G, Lorizzo K, Rotmensz N, Bagnardi V, Cinieri S, Colleoni M, et al. Breast cancer in Hodgkin's disease and non-Hodgkin's lymphoma survivors. Ann Oncol. 2007; 18:288–92.
Article25. Ng AK, Bernardo MP, Weller E, Backstrand KH, Silver B, Marcus KC, et al. Long-term survival and competing causes of death in patients with early-stage Hodgkin's disease treated at age 50 or younger. J Clin Oncol. 2002; 20:2101–8.
Article26. Elkin EB, Klem ML, Gonzales AM, Ishill NM, Hodgson D, Ng AK, et al. Characteristics and outcomes of breast cancer in women with and without a history of radiation for Hodgkin's lymphoma: a multi-institutional, matched cohort study. J Clin Oncol. 2011; 29:2466–73.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Basaloid Squamous Cell Carcinoma Occurring in the Mobile Tongue
- A Case or Squamous Cell Carcinoma of the Renal Pelvis Associated with Squamous Cell Carcinoma in Situ of the Ureter
- Secondary Squamous Cell Carcinoma of the Oral Cavity after Nasopharyngeal Carcinoma
- A Case of Squamous Cell Carcinoma of the Ovary Arising in Mature Cystic Teratoma
- Clinical Outcome of Squamous Cell Carcinoma of the Tongue in Young Patients: A Stage-Matched Comparative Analysis