Transl Clin Pharmacol.
2017 Jun;25(2):93-100. 10.12793/tcp.2017.25.2.93.
Bioequivalence data analysis for the case of separate hospitalization
- Affiliations
-
- 1Department of Clinical Pharmacology and Therapeutics Asan Medical Center, University of Ulsan, Seoul 05505, Republic of Korea. ksbae@amc.seoul.kr
- 2Department of Applied Statistics, Yonsei University, Seoul 03722, Republic of Korea.
- KMID: 2386766
- DOI: http://doi.org/10.12793/tcp.2017.25.2.93
Abstract
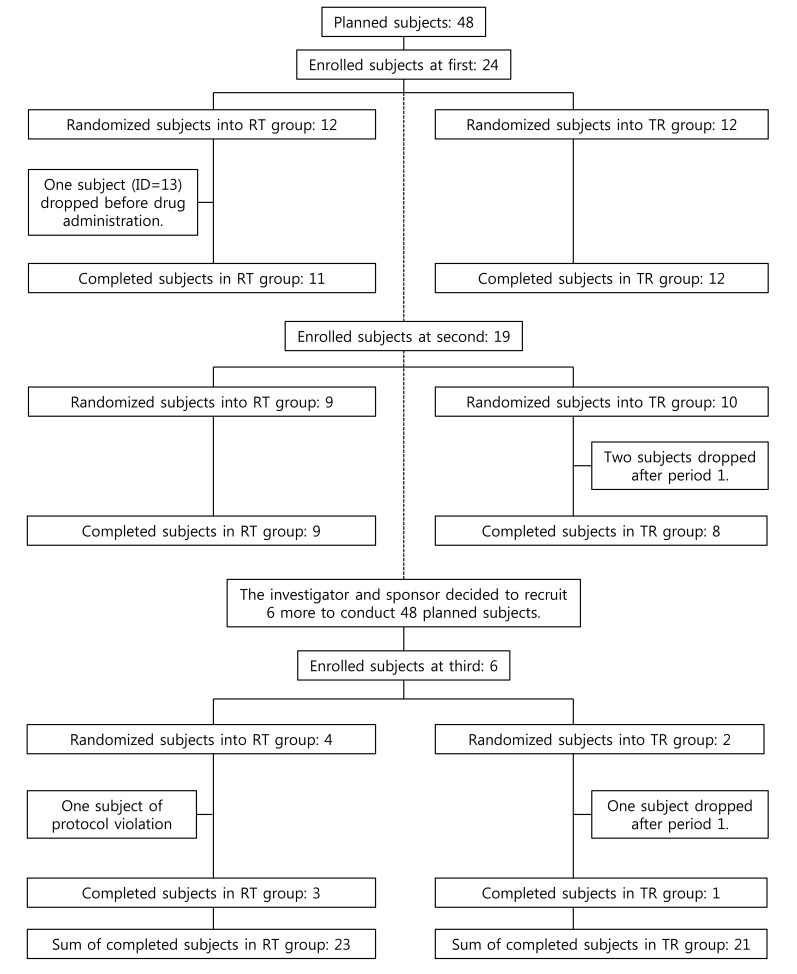
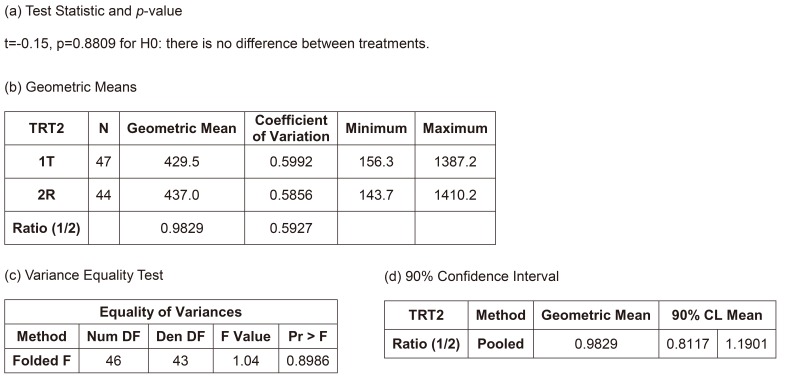
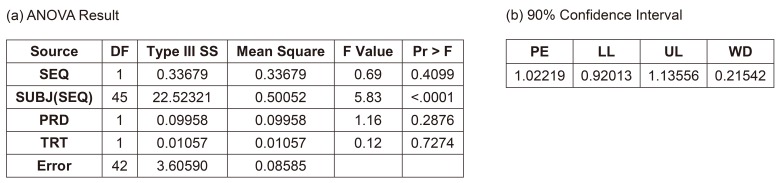
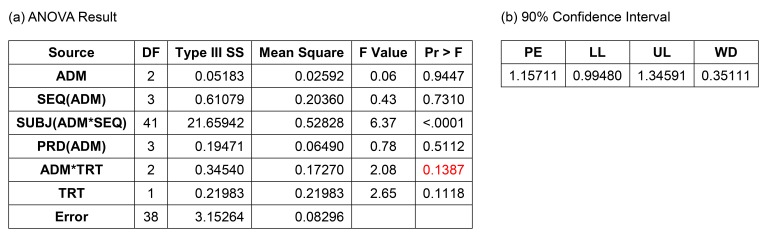
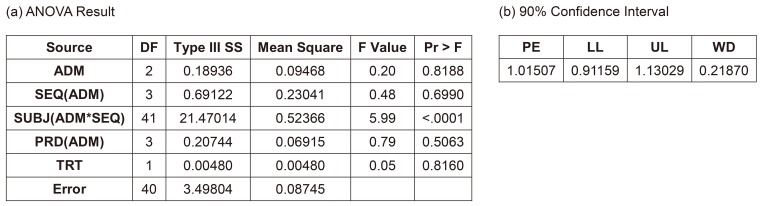
- A bioequivalence study is usually conducted with the same-day drug administration. However, hospitalization is occasionally separated for logistical, operational, or other reasons. Recently, there was a case of separate hospitalization because of difficulties in subject recruitment. This article suggests a better way of bioequivalence data analysis for the case of separate hospitalization. The key features are (1) considering the hospitalization date as a random effect than a fixed effect and 2) using "PROC MIXED" instead of "PROC GLM" to include incomplete subject data.
Figure
Reference
-
1. Park SH. Design of Experiments. 2nd ed. Seoul: Min-Young Sa;2003. p. 58–60. p. 107–109. p. 146–148.2. EMA. Questions & Answers: positions on specific questions addressed to the Pharmacokinetics Working Party (PKWP). 2015. p. 32.Smith MK. Inappropriately Designating a Factor as Fixed or Random. Accessed 1 May 2017. https://www.ma.utexas.edu/users/mks/statmistakes/fixedvsrandom.html.4. SAS. SAS/STAT 9.3 User's Guide. SAS Institute;2011. p. 217–218.5. SAS. SAS/STAT 14.1 User's Guide. SAS Institute;2015. p. 123.6. Elliott AC, Woodward WA. SAS Essentials: Mastering SAS for Data Analytics. 2nd ed. Wiley;2015.7. Galwey NW. Introduction to Mixed Modeling: Beyond Regression and Analysis of Variance. 2nd ed. Wiley;2014.8. Haney SA, Bowman D, Chakravarty A, Davies A, Shamu C. An Introduction to High Content Screening: Imaging Technology, Assay Development, and Data Analysis in Biology and Drug Discovery. Wiley;2015.9. Torbeck LD. Pharmaceutical and Medical Device Validation by Experimental Design. CRC Press;2017.10. Aris VM. 8. Using microarrays to measure cellular changes induced by biomaterials. Characterization of biomaterials. Woodhead Publishing;2012.11. Gibson D. Methods in Comparative Plant Population Ecology. 2nd ed. Oxford University Press;2014.12. Diletti E, Hauschke D, Steinijans VW. Sample size determination for bioequivalence assessment by means of confidence intervals. Int J Clin Pharmacol Ther Toxicol. 1992; 30(Suppl 1):S51–S58. PMID: 1601532.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bioequivalence data analysis
- Bioequivalence Test and Its Significance
- A post hoc analysis of intra-subject coefficients of variation in pharmacokinetic measures to calculate optimal sample sizes for bioequivalence studies
- A post hoc analysis of intra-subject coefficients of variation in pharmacokinetic measures to calculate optimal sample sizes for bioequivalence studies
- Establishment and Significance of Bioequivalence Recommendations for Individual Products-Drugs Acting on Circulatory System and Others