Obstet Gynecol Sci.
2017 Jul;60(4):357-361. 10.5468/ogs.2017.60.4.357.
Atypical squamous cells of undetermined significance and low-grade squamous intraepithelial lesion triage in Korean women: Revisiting the 2012 American Society of Colposcopy and Cervical Pathology screening guidelines
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Guro Hospital, Korea University College of Medicine, Seoul, Korea. jhhong93@korea.ac.kr
- 2Department of Obstetrics and Gynecology, Ansan Hospital, Korea University College of Medicine, Ansan, Korea.
- KMID: 2386277
- DOI: http://doi.org/10.5468/ogs.2017.60.4.357
Abstract
OBJECTIVE
To determine whether triage for atypical squamous cells of undetermined significance (ASC-US) and low-grade squamous intraepithelial lesion (LSIL) from the updated American Society for Colposcopy and Cervical Pathology cervical cancer screening guidelines is applicable in Korean women.
METHODS
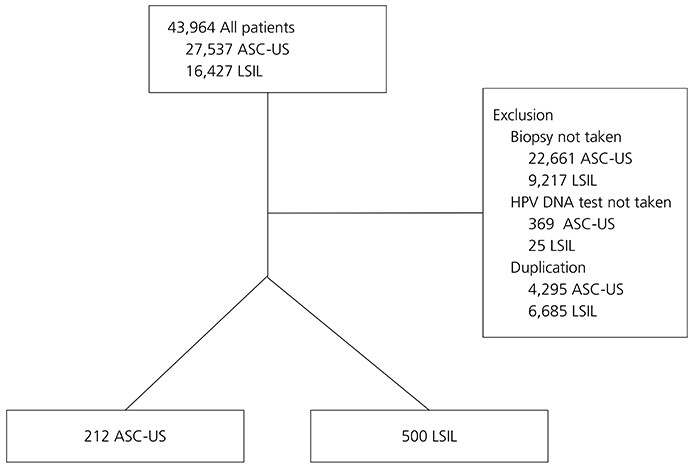
We investigated women with ASC-US or LSIL including referred from local hospitals visited for cervical cancer screening at Korea University Guro Hospital from February 2004 to December 2014. Detailed information on the results of Papanicolaou (Pap) smears, human papillomavirus (HPV) DNA tests, and cervical biopsies were collected through chart review. Cervical biopsy results were compared in eligible women according to individual Pap smear findings and HPV DNA status.
RESULTS
Of 216,723 possible cases, 3,196 were included. There were 212 (6.6%) women with ASC-US and 500 (15.6%) with LSIL. The risk of ≥cervical intraepithelial neoplasia (CIN) 2 was significantly higher in women who were ASC-US/HPV+ than ASC-US/HPV- and LSIL/HPV+ than LSIL/HPV- (93.3% vs. 6.7% and 96.7% vs. 3.3%, P<0.001 and P<0.001, respectively). The risk of ≥CIN 3 was also significantly higher in women who were ASC-US/HPV+ than ASC-US/HPV- and LSIL/HPV+ than LSIL/HPV- (97.0% vs. 3.0% and 93.0% vs. 7.0%, P<0.001 and P<0.001, respectively). Age-stratified analysis revealed that more CIN 2 or CIN 3 was diagnosed in women aged 30 to 70 with ASC-US or LSIL when HPV DNA was present.
CONCLUSION
Observation with Pap and HPV DNA tests rather than immediate colposcopy is a reasonable strategy for ASC-US or LSIL when the HPV DNA test is negative, especially in women aged 30 to 70. Reflection of these results should be considered in future Korean screening guidelines.
Keyword
MeSH Terms
Figure
Reference
-
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.2. Lee YH, Choi KS, Lee HY, Jun JK. Current status of the National Cancer Screening Program for cervical cancer in Korea, 2009. J Gynecol Oncol. 2012; 23:16–21.3. Jung KW, Won YJ, Kong HJ, Oh CM, Cho H, Lee DH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015; 47:127–141.4. Jung KW, Won YJ, Oh CM, Kong HJ, Cho H, Lee DH, et al. Prediction of cancer incidence and mortality in Korea, 2015. Cancer Res Treat. 2015; 47:142–148.5. Seol HJ, Ki KD, Lee JM. Epidemiologic characteristics of cervical cancer in Korean women. J Gynecol Oncol. 2014; 25:70–74.6. Oh CM, Jung KW, Won YJ, Shin A, Kong HJ, Jun JK, et al. Trends in the incidence of in situ and invasive cervical cancer by age group and histological type in Korea from 1993 to 2009. PLoS One. 2013; 8:e72012.7. Lee JK, Hong JH, Kang S, Kim DY, Kim BG, Kim SH, et al. Practice guidelines for the early detection of cervical cancer in Korea: Korean Society of Gynecologic Oncology and the Korean Society for Cytopathology 2012 edition. J Gynecol Oncol. 2013; 24:186–203.8. Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D, et al. 2006 Consensus guidelines for the management of women with abnormal cervical screening tests. J Low Genit Tract Dis. 2007; 11:201–222.9. Partridge EE, Abu-Rustum NR, Campos SM, Fahey PJ, Farmer M, Garcia RL, et al. Cervical cancer screening. J Natl Compr Canc Netw. 2010; 8:1358–1386.10. Moyer VA. U.S. Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012; 156:880–891.11. Massad LS, Einstein MH, Huh WK, Katki HA, Kinney WK, Schiffman M, et al. 2012 Updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013; 121:829–846.12. Lee JK, Kim MK, Song SH, Hong JH, Min KJ, Kim JH, et al. Comparison of human papillomavirus detection and typing by hybrid capture 2, linear array, DNA chip, and cycle sequencing in cervical swab samples. Int J Gynecol Cancer. 2009; 19:266–272.13. Hong JH, Song SH, Kim JK, Han JH, Lee JK. Comparison of the novel human papillomavirus 4 auto-capillary electrophoresis test with the hybrid capture 2 assay and with the PCR HPV typing set test in the detection of high-risk HPV including HPV 16 and 18 genotypes in cervical specimens. J Korean Med Sci. 2009; 24:579–584.14. Moscicki AB, Cox JT. Practice improvement in cervical screening and management (PICSM): symposium on management of cervical abnormalities in adolescents and young women. J Low Genit Tract Dis. 2010; 14:73–80.15. Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999; 189:12–19.16. Coste J, Cochand-Priollet B, de Cremoux P, Le Gales C, Cartier I, Molinie V, et al. Cross sectional study of conventional cervical smear, monolayer cytology, and human papillomavirus DNA testing for cervical cancer screening. BMJ. 2003; 326:733.17. Pedersen K, Burger EA, Sy S, Kristiansen IS, Kim JJ. Cost-effective management of women with minor cervical lesions: revisiting the application of HPV DNA testing. Gynecol Oncol. 2016; 143:326–333.18. Kyrgiou M, Kalliala I, Mitra A, Ng KY, Raglan O, Fotopoulou C, et al. Immediate referral to colposcopy versus cytological surveillance for low-grade cervical cytological abnormalities in the absence of HPV test: a systematic review and a meta-analysis of the literature. Int J Cancer. 2017; 140:216–223.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Visual inspection with acetic acid for detection of high grade lesion in atypical squamous cells and low grade squamous intraepithelial lesions from cervical Pap smear
- Adherence to the American Society for Colposcopy and Cervical Pathology guidelines: an observational study
- Comparison of Qualified Diagnosis of "Atypical Squamous Cells of Undetermined Significance" with Subsequent Biopsy
- The Clinical Significance of Cervical ASCUS(Atypical Squamous Cells of Undetermined Significance) and Its Relationship with Eating Habits in Asymptommatic Women
- Clinical Significance of HPV DNA test for Management of Patients with Diagnosis of Atypical Squamous Cells of Undetermined Significance/Low-grade Squamous Intraepithelial Lesions