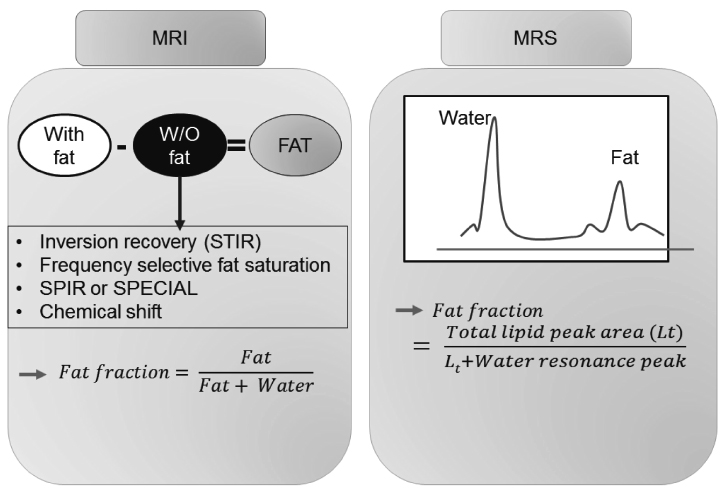
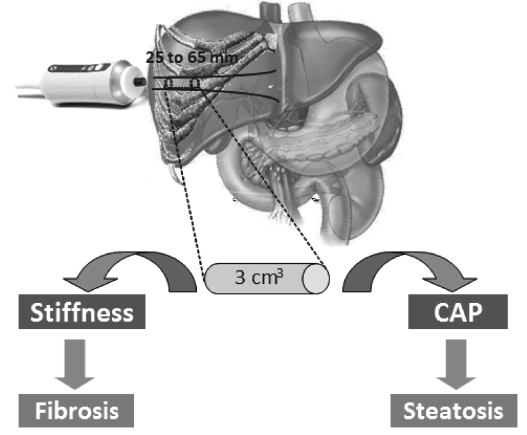
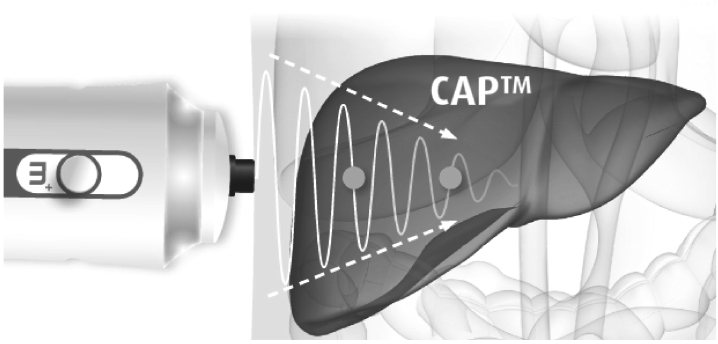
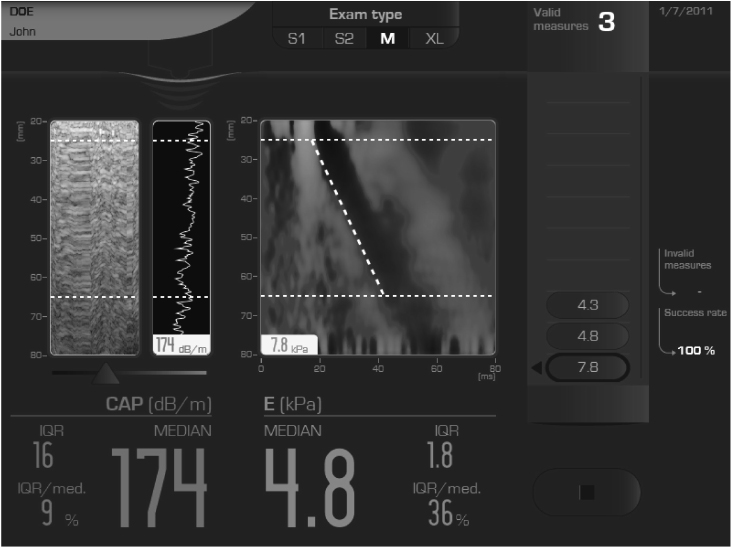
J Korean Diabetes.
2017 Jun;18(2):88-101. 10.4093/jkd.2017.18.2.88.
Radiologic Evaluation of Non-Alcoholic Fatty Liver Disease in Diabetic Patient
- Affiliations
-
- 1Division of Geriatrics, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. cokim@yuhs.ac
- 2Division of Gastroenterology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Radiology, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2385570
- DOI: http://doi.org/10.4093/jkd.2017.18.2.88
Abstract
- Non-alcoholic fatty liver disease (NAFLD), traditionally considered as a disease of hepatologists, has recently become a major concern in patients with type 2 diabetes mellitus (T2DM) as T2DM seems to worsen the course of NAFLD and vice versa. Furthermore, the increasing prevalence of NAFLD in T2DM and the complex mechanisms between these two diseases make physicians caring for patients with T2DM face many uncertainties in the diagnosis of NAFLD. Although the liver biopsy is considered as the gold standard of the diagnosis of NAFLD so far, it has several limitations such as infection, bleeding and cost. Hence, radiologic evaluations have been increasingly accepted as noninvasive alternatives to liver biopsy. Currently, 4 major imaging tools are available for measuring liver fat, including ultrasonography, computed tomography, magnetic resonance imaging and liver fibroscan. This article will describe these methods used to evaluate hepatic steatosis in patients with T2DM, including the diagnostic accuracy, limitations, and practical applicability.
Keyword
MeSH Terms
Figure
Reference
-
1. Clark JM, Diehl AM. Nonalcoholic fatty liver disease: an underrecognized cause of cryptogenic cirrhosis. JAMA. 2003; 289:3000–3004.2. Sattar N, Forrest E, Preiss D. Non-alcoholic fatty liver disease. BMJ. 2014; 349:g4596.
Article3. Marjot T, Cobbold J. Non-alcoholic fatty liver disease. Liver disease in clinical practice. Cham: Springer;2017. p. 111–129.4. Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013; 10:656–665.
Article5. Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013; 10:686–690.
Article6. Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013; 10:330–344.
Article7. Kim SK, Choi YJ, Huh BW, Park SW, Lee EJ, Cho YW, Huh KB. Nonalcoholic fatty liver disease is associated with increased carotid intima-media thickness only in type 2 diabetic subjects with insulin resistance. J Clin Endocrinol Metab. 2014; 99:1879–1884.
Article8. Younossi ZM, Gramlich T, Matteoni CA, Boparai N, McCullough AJ. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol. 2004; 2:262–265.
Article9. Cusi K, Sanyal AJ, Zhang S, Hartman ML, Bue-Valleskey JM, Hoogwerf BJ, Haupt A. Non-alcoholic fatty liver disease (NAFLD) prevalence and its metabolic associations in patients with type 1 diabetes and type 2 diabetes. Diabetes Obes Metab. 2017; DOI: 10.1111/dom.12973. [Epub ahead of print].
Article10. Leite NC, Salles GF, Araujo AL, Villela-Nogueira CA, Cardoso CR. Prevalence and associated factors of nonalcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int. 2009; 29:113–119.
Article11. Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, Zenari L, Falezza G. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005; 54:3541–3546.
Article12. Prashanth M, Ganesh HK, Vima MV, John M, Bandgar T, Joshi SR, Shah SR, Rathi PM, Joshi AS, Thakkar H, Menon PS, Shah NS. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. J Assoc Physicians India. 2009; 57:205–210.13. Gupte P, Amarapurkar D, Agal S, Baijal R, Kulshrestha P, Pramanik S, Patel N, Madan A, Amarapurkar A, Hafeezunnisa . Non-alcoholic steatohepatitis in type 2 diabetes mellitus. J Gastroenterol Hepatol. 2004; 19:854–858.
Article14. Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005; 129:113–121.
Article15. Yoon HJ, Lee Y, Cha BS. Causal relationship of nonalcoholic fatty liver disease with obesity and insulin resistance. J Korean Diabetes. 2014; 15:76–81.
Article16. European Association for the Study of the Liver (EASL). European Association for the Study of Diabetes (EASD). European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016; 64:1388–1402.17. Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines: management of nonalcoholic fatty liver disease. Clin Mol Hepatol. 2013; 19:325–348.18. Nascimbeni F, Pais R, Bellentani S, Day CP, Ratziu V, Loria P, Lonardo A. From NAFLD in clinical practice to answers from guidelines. J Hepatol. 2013; 59:859–871.
Article19. Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012; 55:2005–2023.
Article20. Adams LA, Waters OR, Knuiman MW, Elliott RR, Olynyk JK. NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: an eleven-year follow-up study. Am J Gastroenterol. 2009; 104:861–867.
Article21. Shibata M, Kihara Y, Taguchi M, Tashiro M, Otsuki M. Nonalcoholic fatty liver disease is a risk factor for type 2 diabetes in middle-aged Japanese men. Diabetes Care. 2007; 30:2940–2944.
Article22. Liu M, Wang J, Zeng J, Cao X, He Y. Association of NAFLD with diabetes and the impact of BMI changes: a 5-year cohort study based on 18,507 elderly. J Clin Endocrinol Metab. 2017; 102:1309–1316.
Article23. Yamazaki H, Tsuboya T, Tsuji K, Dohke M, Maguchi H. Independent association between improvement of nonalcoholic fatty liver disease and reduced incidence of type 2 diabetes. Diabetes Care. 2015; 38:1673–1679.
Article24. Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T. LIDO Study Group. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005; 128:1898–1906.
Article25. Wai CT, Tan LH, Kaur M, Da Costa M, Quak SH, Tan KC. Pitfalls in interpreting liver biopsy results: the story of the blind men and the elephant. Liver Transpl. 2002; 8:1200–1201.
Article26. Alkhouri N, Feldstein AE. Noninvasive diagnosis of nonalcoholic fatty liver disease: are we there yet? Metabolism. 2016; 65:1087–1095.
Article27. Lăpădat AM, Jianu IR, Ungureanu BS, Florescu LM, Gheonea DI, Sovaila S, Gheonea IA. Non-invasive imaging techniques in assessing non-alcoholic fatty liver disease: a current status of available methods. J Med Life. 2017; 10:19–26.
Article28. Lee SS, Park SH. Radiologic evaluation of nonalcoholic fatty liver disease. World J Gastroenterol. 2014; 20:7392–7402.
Article29. Hepburn MJ, Vos JA, Fillman EP, Lawitz EJ. The accuracy of the report of hepatic steatosis on ultrasonography in patients infected with hepatitis C in a clinical setting: a retrospective observational study. BMC Gastroenterol. 2005; 5:14.
Article30. de Alwis NM, Anstee QM, Day CP. How to diagnose nonalcoholic fatty liver disease. Dig Dis. 2016; 34:Suppl 1. 19–26.
Article31. Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, Mullen KD, Cooper JN, Sheridan MJ. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002; 123:745–750.
Article32. van Werven JR, Marsman HA, Nederveen AJ, Smits NJ, ten Kate FJ, van Gulik TM, Stoker J. Assessment of hepatic steatosis in patients undergoing liver resection: comparison of US, CT, T1-weighted dual-echo MR imaging, and point-resolved 1H MR spectroscopy. Radiology. 2010; 256:159–168.
Article33. Strauss S, Gavish E, Gottlieb P, Katsnelson L. Interobserver and intraobserver variability in the sonographic assessment of fatty liver. AJR Am J Roentgenol. 2007; 189:W320–W323.
Article34. de Moura Almeida A, Cotrim HP, Barbosa DB, de Athayde LG, Santos AS, Bitencourt AG, de Freitas LA, Rios A, Alves E. Fatty liver disease in severe obese patients: diagnostic value of abdominal ultrasound. World J Gastroenterol. 2008; 14:1415–1418.
Article35. Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011; 140:124–131.
Article36. Musso G, Gambino R, Cassader M, Pagano G. Metaanalysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011; 43:617–649.
Article37. Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, Day C, Arcaro G. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007; 30:1212–1218.
Article38. Lawrence DA, Oliva IB, Israel GM. Detection of hepatic steatosis on contrast-enhanced CT images: diagnostic accuracy of identification of areas of presumed focal fatty sparing. AJR Am J Roentgenol. 2012; 199:44–47.
Article39. Kim DY, Park SH, Lee SS, Kim HJ, Kim SY, Kim MY, Lee Y, Kim TK, Khalili K, Bae MH, Lee JY, Lee SG, Yu ES. Contrast-enhanced computed tomography for the diagnosis of fatty liver: prospective study with sameday biopsy used as the reference standard. Eur Radiol. 2010; 20:359–366.
Article40. Wells MM, Li Z, Addeman B, McKenzie CA, Mujoomdar A, Beaton M, Bird J. Computed tomography measurement of hepatic steatosis: prevalence of hepatic steatosis in a Canadian population. Can J Gastroenterol Hepatol. 2016; 2016:4930987.
Article41. Park YS, Park SH, Lee SS, Kim DY, Shin YM, Lee W, Lee SG, Yu ES. Biopsy-proven nonsteatotic liver in adults: estimation of reference range for difference in attenuation between the liver and the spleen at nonenhanced CT. Radiology. 2011; 258:760–766.
Article42. Patrick D, White FE, Adams PC. Long-term amiodarone therapy: a cause of increased hepatic attenuation on CT. Br J Radiol. 1984; 57:573–576.
Article43. Tota-Maharaj R, Blaha MJ, Zeb I, Katz R, Blankstein R, Blumenthal RS, Budoff MJ, Nasir K. Ethnic and sex differences in fatty liver on cardiac computed tomography: the multi-ethnic study of atherosclerosis. Mayo Clin Proc. 2014; 89:493–503.
Article44. Pickhardt PJ, Park SH, Hahn L, Lee SG, Bae KT, Yu ES. Specificity of unenhanced CT for non-invasive diagnosis of hepatic steatosis: implications for the investigation of the natural history of incidental steatosis. Eur Radiol. 2012; 22:1075–1082.
Article45. Bae JC, Lee WY, Yoon KH, Park JY, Son HS, Han KA, Lee KW, Woo JT, Ju YC, Lee WJ, Cho YY, Lee MK. Improvement of nonalcoholic fatty liver disease with Carnitine-Orotate Complex in Type 2 Diabetes (CORONA): a randomized controlled trial. Diabetes Care. 2015; 38:1245–1252.
Article46. Vu KN, Gilbert G, Chalut M, Chagnon M, Chartrand G, Tang A. MRI-determined liver proton density fat fraction, with MRS validation: comparison of regions of interest sampling methods in patients with type 2 diabetes. J Magn Reson Imaging. 2016; 43:1090–1099.
Article47. Dulai PS, Sirlin CB, Loomba R. MRI and MRE for noninvasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: clinical trials to clinical practice. J Hepatol. 2016; 65:1006–1016.
Article48. Idilman IS, Aniktar H, Idilman R, Kabacam G, Savas B, Elhan A, Celik A, Bahar K, Karcaaltincaba M. Hepatic steatosis: quantification by proton density fat fraction with MR imaging versus liver biopsy. Radiology. 2013; 267:767–775.
Article49. Idilman IS, Keskin O, Celik A, Savas B, Halil Elhan A, Idilman R, Karcaaltincaba M. A comparison of liver fat content as determined by magnetic resonance imagingproton density fat fraction and MRS versus liver histology in non-alcoholic fatty liver disease. Acta Radiol. 2016; 57:271–278.
Article50. Noworolski SM, Tien P, Westphalen A, Merriman R, Vigneron DB, Qayyum A. Mr spectroscopy of nonalcoholic fatty liver disease. Proc Intl Soc Mag Reson Med. 2005; 13:336.51. Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005; 288:E462–E468.
Article52. Tang A, Tan J, Sun M, Hamilton G, Bydder M, Wolfson T, Gamst AC, Middleton M, Brunt EM, Loomba R, Lavine JE, Schwimmer JB, Sirlin CB. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology. 2013; 267:422–431.
Article53. Doycheva I, Cui J, Nguyen P, Costa EA, Hooker J, Hofflich H, Bettencourt R, Brouha S, Sirlin CB, Loomba R. Non-invasive screening of diabetics in primary care for NAFLD and advanced fibrosis by MRI and MRE. Aliment Pharmacol Ther. 2016; 43:83–95.
Article54. Heba ER, Desai A, Zand KA, Hamilton G, Wolfson T, Schlein AN, Gamst A, Loomba R, Sirlin CB, Middleton MS. Accuracy and the effect of possible subject-based confounders of magnitude-based MRI for estimating hepatic proton density fat fraction in adults, using MR spectroscopy as reference. J Magn Reson Imaging. 2016; 43:398–406.
Article55. Permutt Z, Le TA, Peterson MR, Seki E, Brenner DA, Sirlin C, Loomba R. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease-MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther. 2012; 36:22–29.
Article56. Cui J, Philo L, Nguyen P, Hofflich H, Hernandez C, Bettencourt R, Richards L, Salotti J, Bhatt A, Hooker J, Haufe W, Hooker C, Brenner DA, Sirlin CB, Loomba R. Sitagliptin vs. placebo for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol. 2016; 65:369–376.
Article57. Macauley M, Hollingsworth KG, Smith FE, Thelwall PE, Al-Mrabeh A, Schweizer A, Foley JE, Taylor R. Effect of vildagliptin on hepatic steatosis. J Clin Endocrinol Metab. 2015; 100:1578–1585.
Article58. Wong VW, Chan RS, Wong GL, Cheung BH, Chu WC, Yeung DK, Chim AM, Lai JW, Li LS, Sea MM, Chan FK, Sung JJ, Woo J, Chan HL. Community-based lifestyle modification programme for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol. 2013; 59:536–542.
Article59. Raptis DA, Fischer MA, Graf R, Nanz D, Weber A, Moritz W, Tian Y, Oberkofler CE, Clavien PA. MRI: the new reference standard in quantifying hepatic steatosis? Gut. 2012; 61:117–127.
Article60. Averna M. The effect of ezetimibe on NAFLD. Atheroscler Suppl. 2015; 17:27–34.
Article61. de Lédinghen V, Vergniol J, Capdepont M, Chermak F, Hiriart JB, Cassinotto C, Merrouche W, Foucher J, Brigitte le B. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5323 examinations. J Hepatol. 2014; 60:1026–1031.
Article62. Chan WK, Nik Mustapha NR, Mahadeva S. Controlled attenuation parameter for the detection and quantification of hepatic steatosis in nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2014; 29:1470–1476.
Article63. de Lédinghen V, Wong GL, Vergniol J, Chan HL, Hiriart JB, Chan AW, Chermak F, Choi PC, Foucher J, Chan CK, Merrouche W, Chim AM, Le Bail B, Wong VW. Controlled attenuation parameter for the diagnosis of steatosis in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2016; 31:848–855.
Article64. Kikuchi M, Umeda R, Tsuruya K, Shiozawa H, Kikuchi M, Takahashi M, Horie Y, Nishizaki Y. Diagnostic accuracy for non-alcoholic fatty liver disease (NAFLD) with controlled attenuation parameter (CAP) measured by transient elastography. Hepatology. 2015; 62:1269A.65. Chon YE, Jung KS, Kim SU, Park JY, Park YN, Kim DY, Ahn SH, Chon CY, Lee HW, Park Y, Han KH. Controlled attenuation parameter (CAP) for detection of hepatic steatosis in patients with chronic liver diseases: a prospective study of a native Korean population. Liver Int. 2014; 34:102–109.
Article66. Kumar M, Rastogi A, Singh T, Behari C, Gupta E, Garg H, Kumar R, Bhatia V, Sarin SK. Controlled attenuation parameter for non-invasive assessment of hepatic steatosis: does etiology affect performance? J Gastroenterol Hepatol. 2013; 28:1194–1201.
Article67. Shi KQ, Tang JZ, Zhu XL, Ying L, Li DW, Gao J, Fang YX, Li GL, Song YJ, Deng ZJ, Wu JM, Tang KF. Controlled attenuation parameter for the detection of steatosis severity in chronic liver disease: a meta-analysis of diagnostic accuracy. J Gastroenterol Hepatol. 2014; 29:1149–1158.
Article68. Mi YQ, Shi QY, Xu L, Shi RF, Liu YG, Li P, Shen F, Lu W, Fan JG. Controlled attenuation parameter for noninvasive assessment of hepatic steatosis using Fibroscan®: validation in chronic hepatitis B. Dig Dis Sci. 2015; 60:243–251.
Article69. Masaki K, Takaki S, Hyogo H, Kobayashi T, Fukuhara T, Naeshiro N, Honda Y, Nakahara T, Ohno A, Miyaki D, Murakami E, Nagaoki Y, Kawaoka T, Tsuge M, Hiraga N, Hiramatsu A, Imamura M, Kawakami Y, Aikata H, Ochi H, Takahashi S, Arihiro K, Chayama K. Utility of controlled attenuation parameter measurement for assessing liver steatosis in Japanese patients with chronic liver diseases. Hepatol Res. 2013; 43:1182–1189.
Article70. Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Lédinghen V, Kumar M, Lupsor-Platon M, Han KH, Cardoso AC, Ferraioli G, Chan WK, Wong VW, Myers RP, Chayama K, Friedrich-Rust M, Beaugrand M, Shen F, Hiriart JB, Sarin SK, Badea R, Jung KS, Marcellin P, Filice C, Mahadeva S, Wong GL, Crotty P, Masaki K, Bojunga J, Bedossa P, Keim V, Wiegand J. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017; 66:1022–1030.
Article71. Runge JH, Smits L, Verheij J, Nederveen A, Beuers U, Stoker J. 1h-magnetic resonance spectroscopy is superior to controlled attenuation parameter (CAP) in assessing liver fat content in human non-alcoholic fatty liver disease (NAFLD). Hepatology. 2015; 62:1256A.72. Kwok R, Choi KC, Wong GL, Zhang Y, Chan HL, Luk AO, Shu SS, Chan AW, Yeung MW, Chan JC, Kong AP, Wong VW. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut. 2016; 65:1359–1368.
Article73. Ferraioli G, Tinelli C, De Silvestri A, Lissandrin R, Above E, Dellafiore C, Poma G, Di Gregorio M, Maiocchi L, Maserati R, Filice C. The clinical value of controlled attenuation parameter for the noninvasive assessment of liver steatosis. Liver Int. 2016; 36:1860–1866.
Article74. Chon YE, Kim KJ, Jung KS, Kim SU, Park JY, Kim do Y, Ahn SH, Chon CY, Chung JB, Park KH, Bae JC, Han KH. The relationship between type 2 diabetes mellitus and non-alcoholic fatty liver disease measured by controlled attenuation parameter. Yonsei Med J. 2016; 57:885–892.
Article75. Chon YE, Jung KS, Kim KJ, Joo DJ, Kim BK, Park JY, Kim DY, Ahn SH, Han KH, Kim SU. Normal controlled attenuation parameter values: a prospective study of healthy subjects undergoing health checkups and liver donors in Korea. Dig Dis Sci. 2015; 60:234–242.
Article76. Lee YH, Kim JH, Kim SR, Jin HY, Rhee EJ, Cho YM, Lee BW. Lobeglitazone, a novel thiazolidinedione, improves non-alcoholic fatty liver disease in type 2 diabetes: its efficacy and predictive factors related to responsiveness. J Korean Med Sci. 2017; 32:60–69.
Article77. Kinner S, Reeder SB, Yokoo T. Quantitative imaging biomarkers of NAFLD. Dig Dis Sci. 2016; 61:1337–1347.
Article78. Jayakumar S, Harrison SA, Loomba R. Noninvasive markers of fibrosis and inflammation in nonalcoholic fatty liver disease. Curr Hepatol Rep. 2016; 15:86–95.
Article79. Tang A, Chen J, Le TA, Changchien C, Hamilton G, Middleton MS, Loomba R, Sirlin CB. Cross-sectional and longitudinal evaluation of liver volume and total liver fat burden in adults with nonalcoholic steatohepatitis. Abdom Imaging. 2015; 40:26–37.
Article80. Chan WK, Nik Mustapha NR, Wong GL, Wong VW, Mahadeva S. Controlled attenuation parameter using the FibroScan® XL probe for quantification of hepatic steatosis for non-alcoholic fatty liver disease in an Asian population. United European Gastroenterol J. 2017; 5:76–85.
Article81. Sasso M, Audière S, Kemgang A, Gaouar F, Corpechot C, Chazouillères O, Fournier C, Golsztejn O, Prince S, Menu Y, Sandrin L, Miette V. Liver steatosis assessed by controlled attenuation parameter (CAP) measured with the XL probe of the FibroScan: a pilot study assessing diagnostic accuracy. Ultrasound Med Biol. 2016; 42:92–103.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Should you advocate for hepatocellular carcinomasurveillance in patients with alcohol-related liverdisease or non-alcoholic fatty liver disease?
- How to optimize the outcome of liver transplantation for non-alcoholic fatty liver disease
- A Case of Hepatocellular Carcinoma in Non-alcoholic Fatty Liver Disease
- Are patients with alcohol-related fatty liver at increased risk of coronary heart disease?
- Non-invasive imaging biomarkers for liver steatosis in non-alcoholic fatty liver disease: present and future