Clin Endosc.
2017 Mar;50(2):179-184. 10.5946/ce.2016.031.
Efficacy of Albis for the Prevention of Gastric Mucosal Injury Concomitant with the Use of Low-Dose Aspirin: A Prospective, Randomized, Placebo-Controlled Study
- Affiliations
-
- 1Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 2Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 3Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. HJPARK21@yuhs.ac
- 4Department of Internal Medicine, Konkuk University School of Medicine, Seoul, Korea.
- 5Department of Internal Medicine, Soonchunhyang University College of Medicine, Bucheon, Korea.
- KMID: 2383535
- DOI: http://doi.org/10.5946/ce.2016.031
Abstract
- BACKGROUND/AIMS
Long-term use of aspirin can be a risk factor of peptic ulcer diseases. The aim of this study was to evaluate the efficacy of Albis (Daewoong Pharmaceutical Co., Ltd.) for the prevention of gastric mucosal injury caused by aspirin.
METHODS
Aspirin users were enrolled and randomized into the Albis or placebo group. Screening and follow-up endoscopy were performed for modified Lanza scores (MLSs). Primary outcome was measured by the incidence rate of peptic ulcer, and secondary outcomes were measured by the incidence rate of gastritis, improvement in MLS and subjective symptoms.
RESULTS
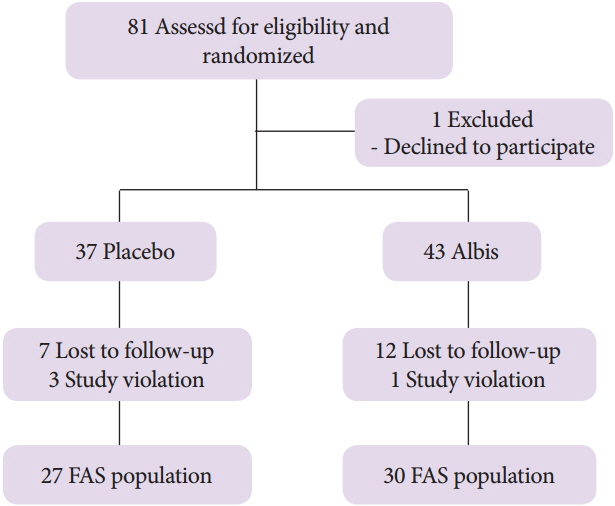
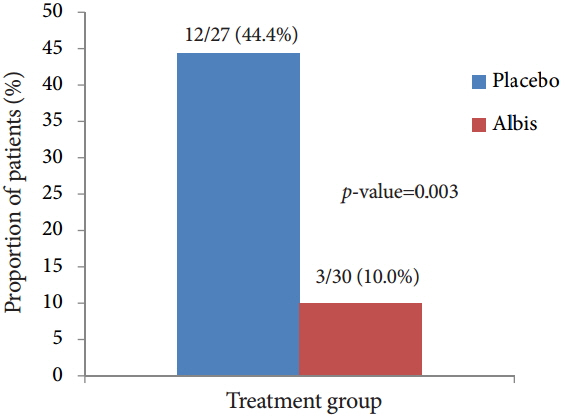
In total, 81 aspirin users were randomized, 43 in the Albis group and 38 in the placebo group. There was no incidence of peptic ulcer in both groups. The incidence of gastritis was significantly higher in the placebo group (44.4% vs. 10.0%, p=0.003); however, the scores of mucosal edema, hyperemia and hemorrhage were not statistically different between the two groups (p>0.05). The frequency of subjective symptoms were more improved in the Albis group than in the placebo group (p=0.023).
CONCLUSIONS
The incidence of gastritis was lower in the group that received low-dose aspirin and Albis. The development of peptic ulcer due to long-term use of aspirin might be prevented with concomitant use of Albis.
Keyword
MeSH Terms
Figure
Reference
-
1. Smith SC Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011; 124:2458–2473.2. Perk J, De Backer G, Gohlke H, et al. European guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012; 33:1635–1701.3. Yeomans ND, Lanas AI, Talley NJ, et al. Prevalence and incidence of gastroduodenal ulcers during treatment with vascular protective doses of aspirin. Aliment Pharmacol Ther. 2005; 22:795–801.
Article4. Sarkar M, Hennessy S, Yang YX. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med. 2008; 149:391–398.
Article5. Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006; 296:2947–2953.
Article6. Ho PM, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA. 2009; 301:937–944.
Article7. Taha AS, McCloskey C, Prasad R, Bezlyak V. Famotidine for the prevention of peptic ulcers and oesophagitis in patients taking low-dose aspirin (FAMOUS): a phase III, randomised, double-blind, placebo-controlled trial. Lancet. 2009; 374:119–125.
Article8. Cook D, Guyatt G, Marshall J, et al. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. N Engl J Med. 1998; 338:791–797.9. Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet. 2009; 374:1449–1461.
Article10. Yeomans N, Lanas A, Labenz J, et al. Efficacy of esomeprazole (20 mg once daily) for reducing the risk of gastroduodenal ulcers associated with continuous use of low-dose aspirin. Am J Gastroenterol. 2008; 103:2465–2473.
Article11. Takeuchi T, Ota K, Harada S, et al. Comparison of teprenone and famotidine against gastroduodenal mucosal damage in patients taking low-dose aspirin. J Gastroenterol Hepatol. 2014; 29 Suppl 4:11–15.
Article12. Naito Y, Yoshikawa T, Iinuma S, et al. Rebamipide protects against indomethacin-induced gastric mucosal injury in healthy volunteers in a double-blind, placebo-controlled study. Dig Dis Sci. 1998; 43(Suppl 9):83S–89S.13. Yamao J, Kikuchi E, Matsumoto M, et al. Assessing the efficacy of famotidine and rebamipide in the treatment of gastric mucosal lesions in patients receiving long-term NSAID therapy (FORCE: famotidine or rebamipide in comparison by endoscopy). J Gastroenterol. 2006; 41:1178–1185.14. Sanuki T, Fujita T, Kutsumi H, et al. Rabeprazole reduces the recurrence risk of peptic ulcers associated with low-dose aspirin in patients with cardiovascular or cerebrovascular disease: a prospective randomized active-controlled trial. J Gastroenterol. 2012; 47:1186–1197.
Article15. Sugano K, Choi MG, Lin JT, et al. Multinational, double-blind, randomised, placebo-controlled, prospective study of esomeprazole in the prevention of recurrent peptic ulcer in low-dose acetylsalicylic acid users: the LAVENDER study. Gut. 2014; 63:1061–1068.
Article16. Sugano K, Matsumoto Y, Itabashi T, et al. Lansoprazole for secondary prevention of gastric or duodenal ulcers associated with long-term low-dose aspirin therapy: results of a prospective, multicenter, double-blind, randomized, double-dummy, active-controlled trial. J Gastroenterol. 2011; 46:724–735.
Article17. Nakashima S, Ota S, Arai S, et al. Usefulness of anti-ulcer drugs for the prevention and treatment of peptic ulcers induced by low doses of aspirin. World J Gastroenterol. 2009; 15:727–731.
Article18. Lanas A, Fuentes J, Benito R, Serrano P, Bajador E, Sáinz R. Helicobacter pylori increases the risk of upper gastrointestinal bleeding in patients taking low-dose aspirin. Aliment Pharmacol Ther. 2002; 16:779–786.
Article19. Lai KC, Lam SK, Chu KM, et al. Lansoprazole for the prevention of recurrences of ulcer complications from long-term low-dose aspirin use. N Engl J Med. 2002; 346:2033–2038.
Article20. Kim SG, Jung HK, Lee HL, et al. Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition. J Gastroenterol Hepatol. 2014; 29:1371–1386.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gastroduodenal Injury in Patients with Low-Dose Enteric Coated Aspirin Treatment
- Efficacy and Safety of Albis(R) in Acute and Chronic Patients with Gastritis: A Double-blind, Placebo-controlled, Randomized Multi-center Study
- Aspirin and Non-steroidal Anti-inflammatory Drugs in Prevention of Gastric Cancer
- Effect of Low-dose, Enteric Coated Aspirin on Gastrointestinal Bleeding in Patients with Coronary Artery Disease
- The Efficacy of Low-dose Aspirin Therapy for Controlled Ovarian Hyperstimulation in IVF-ET