Int J Stem Cells.
2015 Nov;8(2):219-227. 10.15283/ijsc.2015.8.2.219.
Fibrin Scaffolds Designing in order to Human Adipose-derived Mesenchymal Stem Cells Differentiation to Chondrocytes in the Presence of TGF-beta3
- Affiliations
-
- 1Stem Cell laboratory, The Academic Center for Education, Culture and Research, Qom Branch, Qom, Iran. m.ghiasi@acecr.ac.ir
- KMID: 2380816
- DOI: http://doi.org/10.15283/ijsc.2015.8.2.219
Abstract
- BACKGROUND AND OBJECTIVES
One of the most cellular source used for cartilage tissue engineering are mesenchymal stem cells (MSCs). In present study, human MSCs were used as cellular source. Since scaffold plays an important role in tissue engineering the aim of this study is to assess fibrin scaffold ability in chondrogenic differentiation of adipose-derived mesenchymal stem cells (ADMSCs).
METHODS
ADMSCs were isolated and cultured in DMEM medium supplemented with 10% FBS. Also ADMSCs expanded and characterised by flow cytometry. ADMSCs expressed CD44, CD90, CD105 but not CD34. After trypsinization, cells were entered within the fibrin scaffold. Then, chondrogenic medium was added to the scaffold. Seven days after cell culture, cell viability and proliferation were assessed by MTT test. Finally, 14 days after the ending of chondrogenic differentiation, analysis of chondrogenic genes expression was evaluated by RT-PCR and Real time PCR. Also, formation and development of chondrocyte cells was analysed by histological and immunohistochemistry evaluations.
RESULTS
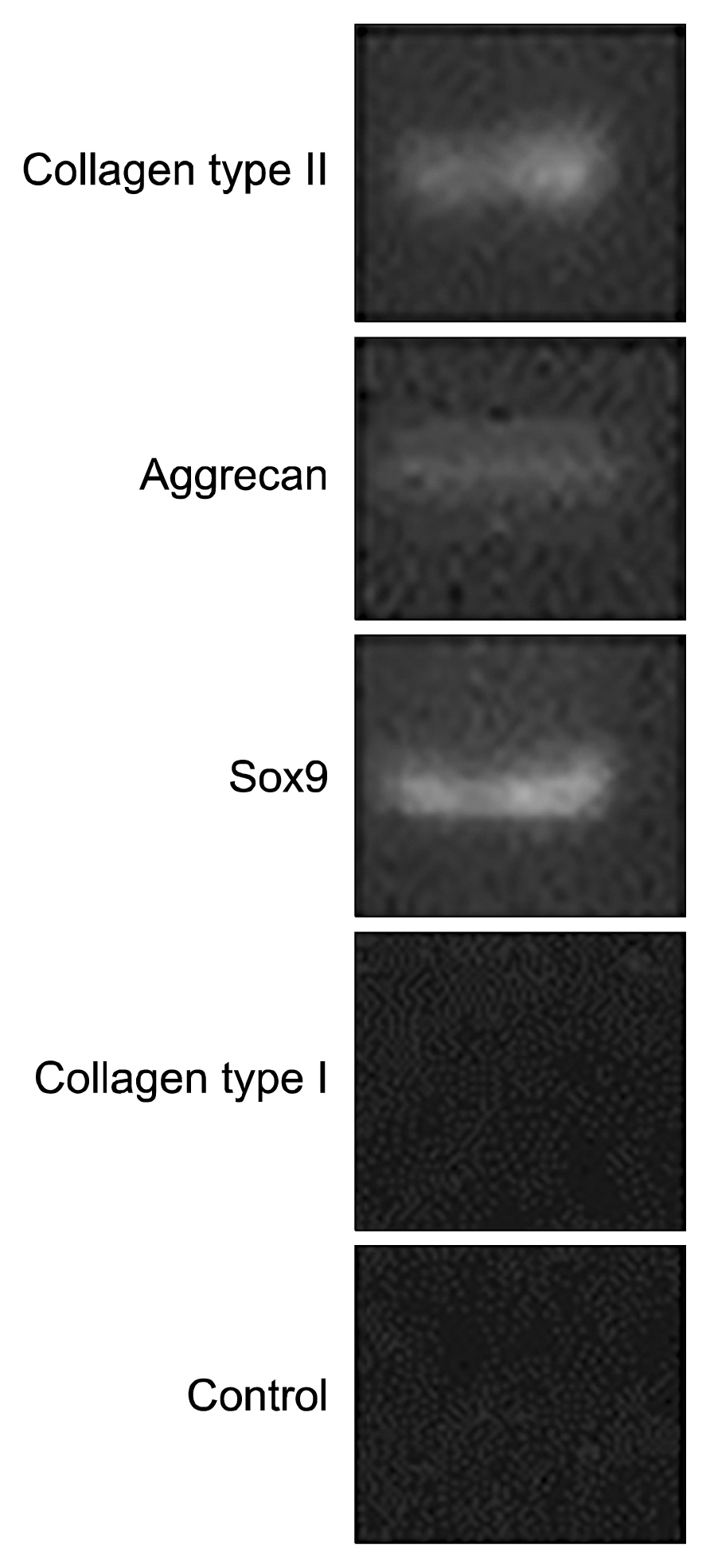
Viability and proliferation as well as chondrogenic genes expression within fibrin scaffold increased significantly compared with control group (cells free scaffold). Also, histological and immunohistochemistry evaluation showed that chondrocyte cells and collagen type II are formed on fibrin scaffold.
CONCLUSIONS
Fibrin is a suitable scaffold for chondrogenic differentiation of ADMSCs.
Keyword
MeSH Terms
-
Cartilage
Cell Culture Techniques
Cell Survival
Chondrocytes*
Collagen Type II
Fibrin*
Flow Cytometry
Humans*
Immunohistochemistry
Mesenchymal Stromal Cells*
Real-Time Polymerase Chain Reaction
Tissue Engineering
Transforming Growth Factor beta3*
Trypsin
Collagen Type II
Fibrin
Transforming Growth Factor beta3
Trypsin
Figure
Reference
-
References
1. Park JG, Lee JH, Kim JN, Kang JA, Kim KJ, Park KD, Han DK, Ahn ST, Rhie JW. Chondrogenic differentiation of human adipose tissue-derived stem cells in functional PLGA scaffolds. Tissue Eng Regen Med. 2011; 8:47–54.2. Wei Y, Hu H, Wang H, Wu Y, Deng L, Qi J. Cartilage regeneration of adipose-derived stem cells in a hybrid scaffold from fibrin-modified PLGA. Cell Transplant. 2009; 18:159–170. DOI: 10.3727/096368909788341261. PMID: 19499704.
Article3. van Osch GJ, Brittberg M, Dennis JE, Bastiaansen-Jenniskens YM, Erben RG, Konttinen YT, Luyten FP. Cartilage repair: past and future--lessons for regenerative medicine. J Cell Mol Med. 2009; 13:792–810. DOI: 10.1111/j.1582-4934.2009.00789.x. PMID: 19453519. PMCID: 3823400.4. Zhang L, Hu J, Athanasiou KA. The role of tissue engineering in articular cartilage repair and regeneration. Crit Rev Biomed Eng. 2009; 37:1–57. DOI: 10.1615/CritRevBiomedEng.v37.i1-2.10. PMID: 20201770. PMCID: 3146065.
Article5. Howard D, Buttery LD, Shakesheff KM, Roberts SJ. Tissue engineering: strategies, stem cells and scaffolds. J Anat. 2008; 213:66–72. DOI: 10.1111/j.1469-7580.2008.00878.x. PMID: 18422523. PMCID: 2475566.
Article6. Mobasheri A, Kalamegam G, Musumeci G, Batt ME. Chondrocyte and mesenchymal stem cell-based therapies for cartilage repair in osteoarthritis and related orthopaedic conditions. Maturitas. 2014; 78:188–198. DOI: 10.1016/j.maturitas.2014.04.017. PMID: 24855933.
Article7. Chang CH, Lin FH, Kuo TF, Liu HC. Cartilage tissue engineering. Biomed Eng Appl Basis Comm. 2005; 17:61–71. DOI: 10.4015/S101623720500010X.
Article8. Rosenbaum AJ, Grande DA, Dines JS. The use of mesenchymal stem cells in tissue engineering: A global assessment. Organogenesis. 2008; 4:23–27. DOI: 10.4161/org.6048. PMID: 19279711. PMCID: 2634175.
Article9. Jacobs SA, Roobrouck VD, Verfaillie CM, Van Gool SW. Immunological characteristics of human mesenchymal stem cells and multipotent adult progenitor cells. Immunol Cell Biol. 2013; 91:32–39. DOI: 10.1038/icb.2012.64. PMID: 23295415. PMCID: 3540326.
Article10. Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007; 100:1249–1260. DOI: 10.1161/01.RES.0000265074.83288.09. PMID: 17495232.
Article11. Chu CR, Friel N. Chondrogenic progenitor cells and articular cartilage repair. Rheumatol Curr Res. 2012; S3:006.12. Diekman BO, Rowland CR, Lennon DP, Caplan AI, Guilak F. Chondrogenesis of adult stem cells from adipose tissue and bone marrow: induction by growth factors and cartilage-derived matrix. Tissue Eng Part A. 2010; 16:523–533. DOI: 10.1089/ten.tea.2009.0398. PMCID: 2813149.
Article13. Sundelacruz S, Kaplan DL. Stem cell- and scaffold-based tissue engineering approaches to osteochondral regenerative medicine. Semin Cell Dev Biol. 2009; 20:646–655. DOI: 10.1016/j.semcdb.2009.03.017. PMID: 19508851. PMCID: 2737137.
Article14. Tsuji W, Rubin JP, Marra KG. Adipose-derived stem cells: Implications in tissue regeneration. World J Stem Cells. 2014; 6:312–321. DOI: 10.4252/wjsc.v6.i3.312. PMID: 25126381. PMCID: 4131273.
Article15. Boehler RM, Graham JG, Shea LD. Tissue engineering tools for modulation of the immune response. Biotechniques. 2011; 51:239–240. 242, 244 passim. PMID: 21988690. PMCID: 3526814.
Article16. Chan BP, Leong KW. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur Spine J. 2008; 17(Suppl 4):467–479. DOI: 10.1007/s00586-008-0745-3. PMID: 19005702. PMCID: 2587658.
Article17. Eyrich D. Fibrin for tissue engineering of cartilage [PhD thesis]. Faculty of Chemistry and Pharmacy;University of Regensburg: 2006.18. Ahmed TA, Dare EV, Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng Part B Rev. 2008; 14:199–215. DOI: 10.1089/ten.teb.2007.0435. PMID: 18544016.
Article19. Ahmed TA, Hincke MT. Fibrin for encapsulation of human mesenchymal stem cells for chondrogenic differentiation. Hayat MA, editor. Stem cells and cancer stem cells. 1st ed. Dordrecht;London: Springer;Netherlands: 2013.
Article20. Ahmed TA, Griffith M, Hincke M. Characterization and inhibition of fibrin hydrogel-degrading enzymes during development of tissue engineering scaffolds. Tissue Eng. 2007; 13:1469–1477. DOI: 10.1089/ten.2006.0354. PMID: 17518706.
Article21. Jockenhoevel S, Zund G, Hoerstrup SP, Chalabi K, Sachweh JS, Demircan L, Messmer BJ, Turina M. Fibrin gel -- advantages of a new scaffold in cardiovascular tissue engineering. Eur J Cardiothorac Surg. 2001; 19:424–430. DOI: 10.1016/S1010-7940(01)00624-8. PMID: 11306307.
Article22. Dew L, MacNeil S, Chong CK. Vascularization strategies for tissue engineers. Regen Med. 2015; 10:211–224. DOI: 10.2217/rme.14.83. PMID: 25835483.
Article23. Laurens N, Koolwijk P, de Maat MP. Fibrin structure and wound healing. J Thromb Haemost. 2006; 4:932–939. DOI: 10.1111/j.1538-7836.2006.01861.x. PMID: 16689737.
Article24. Zisch AH, Schenk U, Schense JC, Sakiyama-Elbert SE, Hubbell JA. Covalently conjugated VEGF--fibrin matrices for endothelialization. J Control Release. 2001; 72:101–113. DOI: 10.1016/S0168-3659(01)00266-8. PMID: 11389989.
Article25. Li J, Pei M. Cell senescence: a challenge in cartilage engineering and regeneration. Tissue Eng Part B Rev. 2012; 18:270–287. DOI: 10.1089/ten.teb.2011.0583. PMID: 22273114.
Article26. Rasmusson I, Ringdén O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res. 2005; 305:33–41. DOI: 10.1016/j.yexcr.2004.12.013. PMID: 15777785.
Article27. Tabera S, Pérez-Simón JA, Díez-Campelo M, Sánchez-Abarca LI, Blanco B, López A, Benito A, Ocio E, Sánchez-Guijo FM, Cañizo C, San Miguel JF. The effect of mesenchymal stem cells on the viability, proliferation and differentiation of B-lymphocytes. Haematologica. 2008; 93:1301–1309. DOI: 10.3324/haematol.12857. PMID: 18641017.
Article28. Park JS, Shim MS, Shim SH, Yang HN, Jeon SY, Woo DG, Lee DR, Yoon TK, Park KH. Chondrogenic potential of stem cells derived from amniotic fluid, adipose tissue, or bone marrow encapsulated in fibrin gels containing TGF-β 3. Biomaterials. 2011; 32:8139–8149. DOI: 10.1016/j.biomaterials.2011.07.043. PMID: 21840589.
Article29. Jo CH, Lee YG, Shin WH, Kim H, Chai JW, Jeong EC, Kim JE, Shim H, Shin JS, Shin IS, Ra JC, Oh S, Yoon KS. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014; 32:1254–1266. DOI: 10.1002/stem.1634. PMID: 24449146.
Article30. Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006; 24:1294–1301. DOI: 10.1634/stemcells.2005-0342. PMID: 16410387.
Article31. Zhu Y, Liu T, Song K, Fan X, Ma X, Cui Z. Adipose-derived stem cell: a better stem cell than BMSC. Cell Biochem Funct. 2008; 26:664–675. DOI: 10.1002/cbf.1488. PMID: 18636461.
Article32. Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, Bunnell BA. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006; 99:1285–1297. DOI: 10.1002/jcb.20904. PMID: 16795045. PMCID: 4048742.
Article33. Mirsaidi A, Kleinhans KN, Rimann M, Tiaden AN, Stauber M, Rudolph KL, Richards PJ. Telomere length, telomerase activity and osteogenic differentiation are maintained in adipose-derived stromal cells from senile osteoporotic SAMP6 mice. J Tissue Eng Regen Med. 2012; 6:378–390. DOI: 10.1002/term.440.
Article34. Murphy JM, Dixon K, Beck S, Fabian D, Feldman A, Barry F. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 2002; 46:704–713. DOI: 10.1002/art.10118. PMID: 11920406.
Article35. Maumus M, Manferdini C, Toupet K, Peyrafitte JA, Ferreira R, Facchini A, Gabusi E, Bourin P, Jorgensen C, Lisignoli G, Noël D. Adipose mesenchymal stem cells protect chondrocytes from degeneration associated with osteoarthritis. Stem Cell Res. 2013; 11:834–844. DOI: 10.1016/j.scr.2013.05.008. PMID: 23811540.
Article36. ter Huurne M, Schelbergen R, Blattes R, Blom A, de Munter W, Grevers LC, Jeanson J, Noël D, Casteilla L, Jorgensen C, van den Berg W, van Lent PL. Antiinflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. Arthritis Rheum. 2012; 64:3604–3613. DOI: 10.1002/art.34626. PMID: 22961401.
Article37. Desando G, Cavallo C, Sartoni F, Martini L, Parrilli A, Veronesi F, Fini M, Giardino R, Facchini A, Grigolo B. Intra-articular delivery of adipose derived stromal cells attenuates osteoarthritis progression in an experimental rabbit model. Arthritis Res Ther. 2013; 15:R22. DOI: 10.1186/ar4156. PMID: 23360790. PMCID: 3672720.
Article38. Toghraie FS, Chenari N, Gholipour MA, Faghih Z, Torabinejad S, Dehghani S, Ghaderi A. Treatment of osteoarthritis with infrapatellar fat pad derived mesenchymal stem cells in Rabbit. Knee. 2011; 18:71–75. DOI: 10.1016/j.knee.2010.03.001.
Article39. Pelaez D, Huang CY, Cheung HS. Cyclic compression maintains viability and induces chondrogenesis of human mesenchymal stem cells in fibrin gel scaffolds. Stem Cells Dev. 2009; 18:93–102. DOI: 10.1089/scd.2008.0030.
Article40. Ho ST, Cool SM, Hui JH, Hutmacher DW. The influence of fibrin based hydrogels on the chondrogenic differentiation of human bone marrow stromal cells. Biomaterials. 2010; 31:38–47. DOI: 10.1016/j.biomaterials.2009.09.021.
Article41. Chung C, Burdick JA. Engineering cartilage tissue. Adv Drug Deliv Rev. 2008; 60:243–262. DOI: 10.1016/j.addr.2007.08.027.
Article42. Xu J, Wang W, Ludeman M, Cheng K, Hayami T, Lotz JC, Kapila S. Chondrogenic differentiation of human mesenchymal stem cells in three-dimensional alginate gels. Tissue Eng Part A. 2008; 14:667–680. DOI: 10.1089/tea.2007.0272. PMID: 18377198.
Article43. Ahmed TA, Giulivi A, Griffith M, Hincke M. Fibrin glues in combination with mesenchymal stem cells to develop a tissue-engineered cartilage substitute. Tissue Eng Part A. 2011; 17:323–335. DOI: 10.1089/ten.tea.2009.0773.
Article44. Awad HA, Wickham MQ, Leddy HA, Gimble JM, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Bio-materials. 2004; 25:3211–3222.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In Vivo Cartilage Formation Using Human Bone Marrow-Derived Mesenchymal Stem Cells Mixed with Fibrin Glue
- Analysis of Molecular Expression in Adipose Tissue-Derived Mesenchymal Stem Cells : Prospects for Use in the Treatment of Intervertebral Disc Degeneration
- Adipose-derived stem cells: characterization and clinical application
- The effect of growth factors on osteogenic differentiation of adipose tissue-derived stromal cells
- Differentiation of adipose-derived stem cells into Schwann-like cells: fetal bovine serum or human serum?