Korean J Ophthalmol.
2017 Jun;31(3):209-216. 10.3341/kjo.2015.0158.
Comparison of Ranibizumab and Bevacizumab for Macular Edema Associated with Branch Retinal Vein Occlusion
- Affiliations
-
- 1Department of Ophthalmology, Kyung Hee University Medical Center, Kyung Hee University School of Medicine, Seoul, Korea. hwkwak@khu.ac.kr
- KMID: 2379877
- DOI: http://doi.org/10.3341/kjo.2015.0158
Abstract
- PURPOSE
To assess the effectiveness and safety of intravitreal ranibizumab compared with bevacizumab for the treatment of macular edema associated with branch retinal vein occlusion (BRVO).
METHODS
This was a retrospective study of 80 eyes with macular edema associated with BRVO. Patients received either 0.5 mg of ranibizumab (n = 24) or 1.25 mg of bevacizumab (n = 56) intravitreally. Both groups received three initial monthly injections followed by as-needed injections. The best-corrected visual acuity, central subfield thickness, mean number of injections, and retreatment rate were evaluated monthly for 6 months after the initial injection.
RESULTS
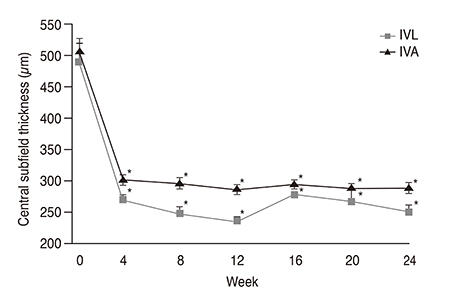
The best-corrected visual acuity significantly improved from logarithm of the minimal angle of resolution (logMAR) 0.55 ± 0.26 at baseline to 0.24 ± 0.26 at 6 months in the ranibizumab group (p < 0.001) and from logMAR 0.58 ± 0.21 at baseline to 0.29 ± 0.25 at 6 months in the bevacizumab group (p < 0.001), which is not a statistically significant difference (p = 0.770). The mean reduction in central subfield thickness at 6 months was 236 ± 164 µm in the ranibizumab group (p < 0.001) and 219 ± 161 µm in the bevacizumab group (p < 0.001), which is not also a statistically significant difference (p = 0.698). The mean numbers of ranibizumab and bevacizumab injections were 3.25 ± 0.53 and 3.30 ± 0.53, respectively (p = 0.602). In addition, after the three initial monthly injections, the retreatment rates for ranibizumab and bevacizumab injections were 20.8% and 26.7%, respectively (p = 0.573).
CONCLUSIONS
Both ranibizumab and bevacizumab were effective for the treatment of BRVO and produced similar visual and anatomic outcomes. In addition, the mean number of injections and the retreatment rates were not significantly different between the groups.
MeSH Terms
Figure
Cited by 1 articles
-
A Comparison of Three Intravitreal Modalities of Branch Retinal Vein Occlusion Macular Edema
Han Song, Hee Jun Song, Ji Ho Yang, Do Gyun Kim
J Korean Ophthalmol Soc. 2018;59(9):834-841. doi: 10.3341/jkos.2018.59.9.834.
Reference
-
1. Klein R, Moss SE, Meuer SM, Klein BE. The 15-year cumulative incidence of retinal vein occlusion: the Beaver Dam Eye Study. Arch Ophthalmol. 2008; 126:513–518.2. Rogers S, McIntosh RL, Cheung N, et al. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010; 117:313–319.e1.3. Christoffersen NL, Larsen M. Pathophysiology and hemodynamics of branch retinal vein occlusion. Ophthalmology. 1999; 106:2054–2062.4. Rehak J, Rehak M. Branch retinal vein occlusion: pathogenesis, visual prognosis, and treatment modalities. Curr Eye Res. 2008; 33:111–131.5. Antonetti DA, Barber AJ, Khin S, et al. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells: Penn State Retina Research Group. Diabetes. 1998; 47:1953–1959.6. Campochiaro PA, Hafiz G, Shah SM, et al. Ranibizumab for macular edema due to retinal vein occlusions: implication of VEGF as a critical stimulator. Mol Ther. 2008; 16:791–799.7. Noma H, Funatsu H, Yamasaki M, et al. Aqueous humour levels of cytokines are correlated to vitreous levels and severity of macular oedema in branch retinal vein occlusion. Eye (Lond). 2008; 22:42–48.8. Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010; 117:1102–1112.e1.9. Yilmaz T, Cordero-Coma M. Use of bevacizumab for macular edema secondary to branch retinal vein occlusion: a systematic review. Graefes Arch Clin Exp Ophthalmol. 2012; 250:787–793.10. Ehlers JP, Decroos FC, Fekrat S. Intravitreal bevacizumab for macular edema secondary to branch retinal vein occlusion. Retina. 2011; 31:1856–1862.11. Narayanan R, Panchal B, Das T, et al. A randomised, double-masked, controlled study of the efficacy and safety of intravitreal bevacizumab versus ranibizumab in the treatment of macular oedema due to branch retinal vein occlusion: MARVEL report no. 1. Br J Ophthalmol. 2015; 99:954–959.12. Argon laser photocoagulation for macular edema in branch vein occlusion: the Branch Vein Occlusion Study Group. Am J Ophthalmol. 1984; 98:271–282.13. Scott IU, Ip MS, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular Edema secondary to branch retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 6. Arch Ophthalmol. 2009; 127:1115–1128.14. Haller JA, Bandello F, Belfort R Jr, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010; 117:1134–1146.e3.15. Kim M, Yu SY, Kim ES, et al. Intravitreal ranibizumab for macular edema secondary to retinal vein occlusion. Ophthalmologica. 2012; 227:132–138.16. Siegel RA, Dreznik A, Mimouni K, et al. Intravitreal bevacizumab treatment for macular edema due to branch retinal vein occlusion in a clinical setting. Curr Eye Res. 2012; 37:823–829.17. Bakri SJ, Snyder MR, Reid JM, et al. Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology. 2007; 114:2179–2182.18. Bakri SJ, Snyder MR, Reid JM, et al. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology. 2007; 114:855–859.19. Mordenti J, Cuthbertson RA, Ferrara N, et al. Comparisons of the intraocular tissue distribution, pharmacokinetics, and safety of 125I-labeled full-length and Fab antibodies in rhesus monkeys following intravitreal administration. Toxicol Pathol. 1999; 27:536–544.20. Terasaki H, Sakamoto T, Shirasawa M, et al. Penetration of bevacizumab and ranibizumab through retinal pigment epithelial layer in vitro. Retina. 2015; 35:1007–1015.21. Papadopoulos N, Martin J, Ruan Q, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF trap, ranibizumab and bevacizumab. Angiogenesis. 2012; 15:171–185.22. Yu L, Liang XH, Ferrara N. Comparing protein VEGF inhibitors: in vitro biological studies. Biochem Biophys Res Commun. 2011; 408:276–281.23. Berg K, Pedersen TR, Sandvik L, Bragadottir R. Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology. 2015; 122:146–152.24. CATT Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011; 364:1897–1908.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Efficacy of Intravitreal Bevacizumab in the Treatment of Macular Edema
- Effects of Intravitreal Bevacizumab Injection in 3 Types of Macular Edema Secondary to Branch Retinal Vein Occlusion
- Two Cases of Macular Edema Associated with Extramacular Branch Retinal Vein Occlusion
- Short-term Effects of Intravitreal Bevacizumab Injection and Macular Edema Patterns in Branch Retinal Vein Occlusion
- Factors Related to Repeatability of Intravitreal Bevacizumab Injections in Branch Retinal Vein Occlusion Macular Edema