J Korean Med Sci.
2017 Jun;32(6):954-960. 10.3346/jkms.2017.32.6.954.
The Rate of Drug-Resistant Tuberculosis in Korean Children and Adolescents Since 2007
- Affiliations
-
- 1Department of Pediatrics, Eulji General Hospital, Eulji University, Seoul, Korea. aym3216@eulji.ac.kr
- 2Department of Pediatrics, Eulji University School of Medicine, Daejeon, Korea.
- 3Korean National Tuberculosis Association, Seoul, Korea.
- 4The Korean Institute of Tuberculosis, Cheongju, Korea.
- KMID: 2377725
- DOI: http://doi.org/10.3346/jkms.2017.32.6.954
Abstract
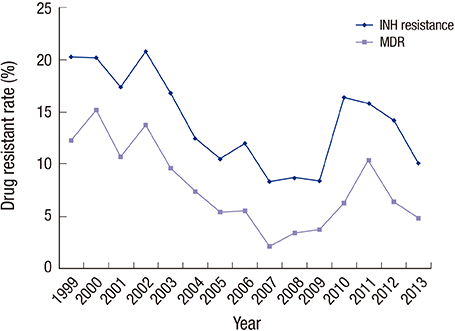
- The incidence of drug-resistant tuberculosis (DR-TB) in pediatric populations is a critical indicator of national TB management and treatment strategies. Limited data exist regarding the rate of pediatric DR-TB. In this study, we aimed to analyze the status of DR-TB in Korean children from 2007 to 2013. We analyzed specimens submitted to the Korean Institute of Tuberculosis using Mycobacterium tuberculosis culture and drug susceptibility tests (DSTs) from January 2007 through December 2013. Specimens from patients ≤ 19 years of age were included. Among the 2,690 cases, 297 cases were excluded because of insufficient data, leaving 2,393 cases for the final analysis. In total, resistance to one or more TB drugs was 13.5%. The resistance rates of each of the drugs were as follows: isoniazid (INH) 10.2%, rifampin (RFP) 5.1%, ethambutol (EMB) 3.7%, and pyrazinamide (PZA) 3.1%. The resistance rate of multidrug-resistant TB (MDR-TB) was 4.2%, and that of extensively drug-resistant TB (XDR-TB) was 0.8%. The overall drug resistance rate demonstrated significant increase throughout the study period (P < 0.001) but showed no significant difference compared to previous study from 1999 to 2007. The drug resistance rate of PZA in ≤ 15 years of age group was significantly greater than that of > 15 years (P < 0.001). The drug resistance rate has increased throughout the study period.
Keyword
MeSH Terms
Figure
Reference
-
1. World Health Organization. Global Tuberculosis Report 2012. Geneva: World Health Organization;2012.2. World Health Organization. Global Tuberculosis Control: WHO Report 2010. Geneva: World Health Organization;2010.3. Park YS, Hong SJ, Boo YK, Hwang ES, Kim HJ, Cho SH, Na KI, Cho EH, Shin SS. The national status of tuberculosis using nationwide medical records survey of patients with tuberculosis in Korea. Tuberc Respir Dis. 2012; 73:48–55.4. Kim SY, Kim HJ, Kim CK, Yoon HR, Bae HG, Lee SH, Sung N, Kim DY, Lee GY, Cho YS, et al. The recent status of multidrug- and extensively drug-resistant tuberculosis in Korea. Tuberc Respir Dis. 2010; 68:146–154.5. Park YK, Park YS, Na KI, Cho EH, Shin SS, Kim HJ. Increased tuberculosis burden due to demographic transition in Korea from 2001 to 2010. Tuberc Respir Dis. 2013; 74:104–110.6. Korea Center for Disease Control and Prevention. Annual Report on the Notified Tuberculosis in Korea 2013. Cheongwon: Korea Centers for Disease Control and Prevention;2013.7. Kim SJ, Bai GH, Hong YP. Drug-resistant tuberculosis in Korea, 1994. Int J Tuberc Lung Dis. 1997; 1:302–308.8. Pablos-Méndez A, Raviglione MC, Laszlo A, Binkin N, Rieder HL, Bustreo F, Cohn DL, Lambregts-van Weezenbeek CS, Kim SJ, Chaulet P, et al. Global surveillance for antituberculosis-drug resistance, 1994–1997. World Health Organization-International Union against Tuberculosis and Lung Disease Working Group on Anti-Tuberculosis Drug Resistance Surveillance. N Engl J Med. 1998; 338:1641–1649.9. Mukherjee JS, Rich ML, Socci AR, Joseph JK, Virú FA, Shin SS, Furin JJ, Becerra MC, Barry DJ, Kim JY, et al. Programmes and principles in treatment of multidrug-resistant tuberculosis. Lancet. 2004; 363:474–481.10. Centers for Disease Control and Prevention. Revised definition of extensively drug-resistant tuberculosis. MMWR Morb Mortal Wkly Rep. 2006; 55:1176.11. Gandhi NR, Nunn P, Dheda K, Schaaf HS, Zignol M, van Soolingen D, Jensen P, Bayona J. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet. 2010; 375:1830–1843.12. Son CH, Yang DK, Rho MS, Jeong JS, Lee H, Lee KN, Choi PJ, Lee SK, Chang KY, Choi IS. Prevalence of drug-resistances in patients with pulmonary tuberculosis and its association with clinical characteristics at one tertiary rcferral hospital in Pusan, Korea. Tuberc Respir Dis. 2001; 51:416–425.13. World Health Organization. Guidelines for the Programmatic Management of Drug-resistant Tuberculosis: Emergency Update 2008. Geneva: World Health Organization;2008.14. Joint Committee for the Revision of Korean Guidelines for Tuberculosis. Korea Centers for Disease Control and Prevention. Korean Guidelines for Tuberculosis. 2nd ed. Cheongju: Korea Centers for Disease Control and Prevention;2014.15. Jeong SH, Lee DD, Choi JC, Kim S, Shin JH, Jeong J, Lee EY, Oh SH, Bai GH, Chang CL. Multi-center study on cost effectiveness of anti-tuberculosis drug susceptibility test. Infect Chemother. 2005; 37:16–21.16. Sass P. Tuberculosis infection and disease in children. Am Fam Physician. 1996; 53:2087–2094.17. de Charnace G, Delacourt C. Diagnostic techniques in paediatric tuberculosis. Paediatr Respir Rev. 2001; 2:120–126.18. Nelson LJ, Wells CD. Global epidemiology of childhood tuberculosis. Int J Tuberc Lung Dis. 2004; 8:636–647.19. Lee SJ, Ahn YM, Kim HJ. Drug resistance of Mycobacterium tuberculosis in children. Korean J Pediatr. 2009; 52:61–67.20. Steiner M, Cosio A. Primary tuberculosis in children. 1. Incidence of primary drug-resistant disease in 332 children observed between the years 1961 and 1964 at the Kings County Medical Center of Brooklyn. N Engl J Med. 1966; 274:755–759.21. Schaaf HS, Gie RP, Beyers N, Sirgel FA, de Klerk PJ, Donald PR. Primary drug-resistant tuberculosis in children. Int J Tuberc Lung Dis. 2000; 4:1149–1155.22. Dye C, Espinal MA, Watt CJ, Mbiaga C, Williams BG. Worldwide incidence of multidrug-resistant tuberculosis. J Infect Dis. 2002; 185:1197–1202.23. Canetti G, Fox W, Khomenko A, Mahler HT, Menon NK, Mitchison DA, Rist N, Smelev NA. Advances in techniques of testing mycobacterial drug sensitivity, and the use of sensitivity tests in tuberculosis control programmes. Bull World Health Organ. 1969; 41:21–43.24. World Health Organization. Companion Handbook to the WHO Guidelines for the Programmatic Management of Drug-resistant Tuberculosis. Geneva: World Health Organization;2014.25. Wayne LG. Simple pyrazinamidase and urease tests for routine identification of mycobacteria. Am Rev Respir Dis. 1974; 109:147–151.26. Korea Center for Disease Control and Prevention. Annual Report on the Notified Tuberculosis in Korea 2014. Cheongju: Korea Centers for Disease Control and Prevention;2014.27. Guo Q, Pan Y, Yang Z, Liu R, Xing L, Peng Z, Zhu C. Epidemiology and clinical characteristics of pediatric drug-resistant tuberculosis in Chongqing, China. PLoS One. 2016; 11:e0151303.28. Dodd PJ, Sismanidis C, Seddon JA. Global burden of drug-resistant tuberculosis in children: a mathematical modelling study. Lancet Infect Dis. 2016; 16:1193–1201.29. Lee HY, Lee J, Lee YS, Kim MY, Lee HK, Lee YM, Shin JH, Ko Y. Drug-resistance pattern of Mycobacterium tuberculosis strains from patients with pulmonary and extrapulmonary tuberculosis during 2006 to 2013 in a Korean tertiary medical center. Korean J Intern Med. 2015; 30:325–334.30. Sheen P, Lozano K, Gilman RH, Valencia HJ, Loli S, Fuentes P, Grandjean L, Zimic M. pncA gene expression and prediction factors on pyrazinamide resistance in Mycobacterium tuberculosis . Tuberculosis (Edinb). 2013; 93:515–522.31. Whitfield MG, Streicher EM, Dolby T, Simpson JA, Sampson SL, Van Helden PD, Van Rie A, Warren RM. Prevalence of pyrazinamide resistance across the spectrum of drug resistant phenotypes of Mycobacterium tuberculosis . Tuberculosis (Edinb). 2016; 99:128–130.32. Kim J, Park YJ, Lee NY, Chang CL, Lee MA, Shin JH. Anti-tuberculosis drug resistant rates in Mycobacterium tuberculosis isolated from respiratory specimens: a multicenter study in Korea. Ann Clin Microbiol. 2013; 16:1–7.33. Kang YA, Choi YJ, Cho YJ, Lee SM, Yoo CG, Kim YW, Han SK, Shim YS, Yim JJ. Cost of treatment for multidrug-resistant tuberculosis in South Korea. Respirology. 2006; 11:793–798.