Korean J Ophthalmol.
2016 Aug;30(4):265-271. 10.3341/kjo.2016.30.4.265.
High Dose Intravitreal Bevacizumab for Refractory Pigment Epithelial Detachment in Age-related Macular Degeneration
- Affiliations
-
- 1Retina Center, Nune Eye Hospital, Seoul, Korea. owkwon0301@yuhs.ac
- KMID: 2373986
- DOI: http://doi.org/10.3341/kjo.2016.30.4.265
Abstract
- PURPOSE
Intravitreal anti-vascular endothelial growth factor (anti-VEGF) is the first choice of treatment for age-related macular degeneration. However, quite a few eyes treated using conventional dose anti-VEGF (CDAV) have persistent pigment epithelial detachment (PED) on optical coherence tomography. This study investigated the efficacy and safety of high dose anti-VEGF (HDAV) for refractory PED.
METHODS
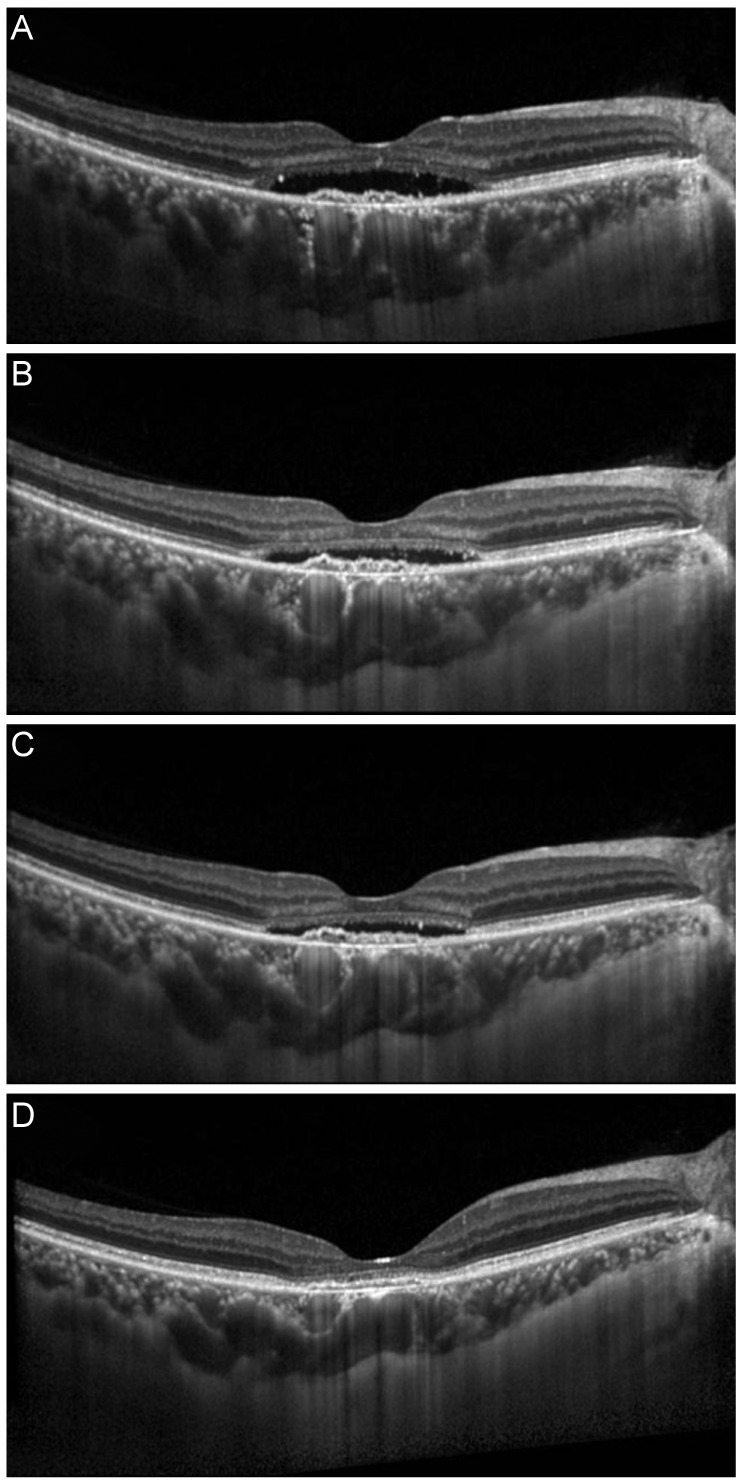
In this retrospective study, 31 eyes of neovascular age-related macular degeneration patients with persistent PED findings despite six or more intravitreal injections of CDAV (bevacizumab 1.25 mg or ranibizumab 2.5 mg) were analyzed. Changes in visual outcome, central foveal thickness, and PED height were compared before and after HDAV (bevacizumab 5.0 mg) for these refractory PED cases.
RESULTS
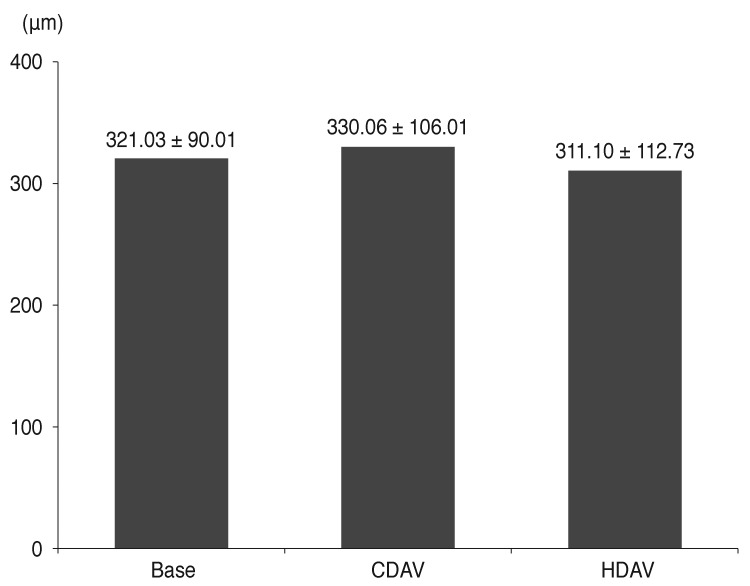
The mean age of patients was 67.7 years. The number of CDAV injections was 12.1. The number of HDAV injections was 3.39. Best-corrected visual acuity in logarithm of the minimum angle of resolution before and after HDAV was 0.49 and 0.41 (p < 0.001), respectively. Central foveal thickness before and after HDAV was 330.06 and 311.10 µm (p = 0.125), respectively. PED height before and after HDAV was 230.28 and 204.07 µm (p = 0.014), respectively. There were no serious adverse reactions in all the eyes.
CONCLUSIONS
Increasing the dose of bevacizumab in refractory PED may be a possible treatment option.
Keyword
MeSH Terms
-
Aged
Angiogenesis Inhibitors/administration & dosage
Bevacizumab/*administration & dosage
Dose-Response Relationship, Drug
Female
Fluorescein Angiography
Fundus Oculi
Humans
Intravitreal Injections
Macular Degeneration/*complications/diagnosis/drug therapy
Male
Middle Aged
Retinal Detachment/diagnosis/*drug therapy/etiology
Retinal Pigment Epithelium/*diagnostic imaging/drug effects
Retrospective Studies
Tomography, Optical Coherence
Vascular Endothelial Growth Factor A/antagonists & inhibitors
Angiogenesis Inhibitors
Bevacizumab
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004; 137:486–495. PMID: 15013873.
Article2. Bressler NM, Bressler SB, Congdon NG, et al. Potential public health impact of Age-Related Eye Disease Study results: AREDS report no. 11. Arch Ophthalmol. 2003; 121:1621–1624. PMID: 14609922.3. Ferris FL 3rd, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984; 102:1640–1642. PMID: 6208888.
Article4. Amin R, Puklin JE, Frank RN. Growth factor localization in choroidal neovascular membranes of age-related macular degeneration. Invest Ophthalmol Vis Sci. 1994; 35:3178–3188. PMID: 7519180.5. Kliffen M, Sharma HS, Mooy CM, et al. Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol. 1997; 81:154–162. PMID: 9059252.
Article6. Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006; 113:363–372.e5. PMID: 16458968.
Article7. Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007; 143:566–583. PMID: 17386270.
Article8. Chan CK, Meyer CH, Gross JG, et al. Retinal pigment epithelial tears after intravitreal bevacizumab injection for neovascular age-related macular degeneration. Retina. 2007; 27:541–551. PMID: 17558314.
Article9. CATT Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011; 364:1897–1908. PMID: 21526923.
Article10. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–1444. PMID: 17021319.
Article11. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–1431. PMID: 17021318.
Article12. Gass JD. Serous retinal pigment epithelial detachment with a notch: a sign of occult choroidal neovascularization. Retina. 1984; 4:205–220. PMID: 6085179.13. Gass JD. Pathogenesis of tears of the retinal pigment epithelium. Br J Ophthalmol. 1984; 68:513–519. PMID: 6204685.
Article14. Brown DM, Michels M, Kaiser PK, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology. 2009; 116:57–65.e5. PMID: 19118696.
Article15. Gaudreault J, Fei D, Rusit J, et al. Preclinical pharmacokinetics of Ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005; 46:726–733. PMID: 15671306.
Article16. Pauleikhoff D, Loffert D, Spital G, et al. Pigment epithelial detachment in the elderly: clinical differentiation, natural course and pathogenetic implications. Graefes Arch Clin Exp Ophthalmol. 2002; 240:533–538. PMID: 12136282.17. Casswell AG, Kohen D, Bird AC. Retinal pigment epithelial detachments in the elderly: classification and outcome. Br J Ophthalmol. 1985; 69:397–403. PMID: 2408659.
Article18. Inoue M, Arakawa A, Yamane S, Kadonosono K. Variable response of vascularized pigment epithelial detachments to ranibizumab based on lesion subtypes, including polypoidal choroidal vasculopathy. Retina. 2013; 33:990–997. PMID: 23446653.
Article19. Parodi MB, Iacono P, Papayannis A, et al. Intravitreal ranibizumab for pigment epithelium detachment with subfoveal occult choroidal neovascularization: a prospective 24-month case series. Am J Ophthalmol. 2013; 155:103–108.e2. PMID: 23022164.
Article20. Bolz M, Michels S, Geitzenauer W, et al. Effect of systemic bevacizumab therapy on retinal pigment epithelial detachment. Br J Ophthalmol. 2007; 91:785–789. PMID: 17050580.
Article21. Chen E, Kaiser RS, Vander JF. Intravitreal bevacizumab for refractory pigment epithelial detachment with occult choroidal neovascularization in age-related macular degeneration. Retina. 2007; 27:445–450. PMID: 17420696.
Article22. Ach T, Hoeh AE, Ruppenstein M, et al. Intravitreal bevacizumab in vascular pigment epithelium detachment as a result of subfoveal occult choroidal neovascularization in age-related macular degeneration. Retina. 2010; 30:1420–1425. PMID: 20543764.
Article23. Modarres M, Naseripour M, Falavarjani KG, et al. Intravitreal injection of 2.5 mg versus 1.25 mg bevacizumab (Avastin) for treatment of CNV associated with AMD. Retina. 2009; 29:319–324. PMID: 19287288.
Article24. Wu L, Arevalo JF, Roca JA, et al. Comparison of two doses of intravitreal bevacizumab (Avastin) for treatment of macular edema secondary to branch retinal vein occlusion: results from the Pan-American Collaborative Retina Study Group at 6 months of follow-up. Retina. 2008; 28:212–219. PMID: 18301025.25. Busbee BG, Ho AC, Brown DM, et al. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2013; 120:1046–1056. PMID: 23352196.
Article26. Costa RA, Jorge R, Calucci D, et al. Intravitreal bevacizumab for choroidal neovascularization caused by AMD (IBeNA Study): results of a phase 1 dose-escalation study. Invest Ophthalmol Vis Sci. 2006; 47:4569–4578. PMID: 17003454.
Article27. Chan CK, Abraham P, Sarraf D. High-dose ranibizumab therapy for vascularized pigment epithelial detachment. Eye (Lond). 2012; 26:882–885. PMID: 22576827.
Article28. Brown DM, Chen E, Mariani A, et al. Super-dose anti-VEGF (SAVE) trial: 2.0 mg intravitreal ranibizumab for recalcitrant neovascular macular degeneration-primary end point. Ophthalmology. 2013; 120:349–354. PMID: 23131717.
Article29. Rosenfeld PJ, Heier JS, Hantsbarger G, Shams N. Tolerability and efficacy of multiple escalating doses of ranibizumab (Lucentis) for neovascular age-related macular degeneration. Ophthalmology. 2006; 113:623.e1. PMID: 16581423.
Article30. Manzano RP, Peyman GA, Khan P, Kivilcim M. Testing intravitreal toxicity of bevacizumab (Avastin). Retina. 2006; 26:257–261. PMID: 16508423.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of High-dose Intravitreal Bevacizumab Injection on Refractory Idiopathic Choroidal Neovasculariz
- Predictors of Retinal Pigment Epithelial Tears in Exudative Macular Degeneration with High Pigment Epithelial Detachment
- Intravitreal Aflibercept for Neovascular Age-Related Macular Degeneration Resistant to Bevacizumab and Ranibizumab
- Effects and Prognostic Factors of Intravitreal Bevacizumab Injection on Choroidal Neovascularization from Age-Related Macular Degeneration
- Combination Treatment for Choroidal Neovascularization Associated With Large Retinal Pigment Epithelial Detachment