J Bone Metab.
2017 Feb;24(1):1-8. 10.11005/jbm.2017.24.1.1.
Role of the Cytokine-like Hormone Leptin in Muscle-bone Crosstalk with Aging
- Affiliations
-
- 1Department of Cellular Biology and Anatomy, Medical College of Georgia, Augusta University, Augusta, GA, USA. mhamrick@augusta.edu
- KMID: 2371826
- DOI: http://doi.org/10.11005/jbm.2017.24.1.1
Abstract
- The cytokine-like hormone leptin is a classic adipokine that is secreted by adipocytes, increases with weight gain, and decreases with weight loss. Additional studies have, however, shown that leptin is also produced by skeletal muscle, and leptin receptors are abundant in both skeletal muscle and bone-derived mesenchymal (stromal) stem cells. These findings suggest that leptin may play an important role in muscle-bone crosstalk. Leptin treatment in vitro increases the expression of myogenic genes in primary myoblasts, and leptin treatment in vivo increases the expression of microRNAs involved in myogenesis. Bone marrow adipogenesis is associated with low bone mass in humans and rodents, and leptin can reduce marrow adipogenesis centrally through its receptors in the hypothalamus as well as directly via its receptors in bone marrow stem cells. Yet, central leptin resistance can increase with age, and low circulating levels of leptin have been observed among the frail elderly. Thus, aging appears to significantly alter leptin-mediated crosstalk among various organs and tissues. Aging is associated with bone loss and muscle atrophy, contributing to frailty, postural instability, and the incidence of falls. Therapeutic interventions such as protein and amino acid supplementation that can increase muscle mass and muscle-derived leptin may have multiple benefits for the elderly that can potentially reduce the incidence of falls and fractures.
MeSH Terms
-
Accidental Falls
Adipocytes
Adipogenesis
Adipokines
Aged
Aging*
Bone Marrow
Frail Elderly
Humans
Hypothalamus
In Vitro Techniques
Incidence
Insulin-Like Growth Factor I
Leptin*
Mesenchymal Stromal Cells
MicroRNAs
Muscle Development
Muscle, Skeletal
Muscular Atrophy
Myoblasts
Osteoporosis
Receptors, Leptin
Rodentia
Sarcopenia
Stem Cells
Weight Gain
Weight Loss
Adipokines
Insulin-Like Growth Factor I
Leptin
MicroRNAs
Receptors, Leptin
Figure
Reference
-
1. Kim SH, Meehan JP, Blumenfeld T, et al. Hip fractures in the United States: 2008 nationwide emergency department sample. Arthritis Care Res (Hoboken). 2012; 64:751–757. PMID: 22190474.
Article2. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001; 56:M146–M156. PMID: 11253156.
Article3. Dawson-Hughes B, Bischoff-Ferrari H. Considerations concerning the definition of sarcopenia. Osteoporos Int. 2016; 27:3139–3144. PMID: 27329218.
Article4. Järvinen TL, Sievanen H, Khan KM, et al. Shifting the focus in fracture prevention from osteoporosis to falls. BMJ. 2008; 336:124–126. PMID: 18202065.
Article5. Fiuza-Luces C, Garatachea N, Berger NA, et al. Exercise is the real polypill. Physiology (Bethesda). 2013; 28:330–358. PMID: 23997192.
Article7. Hamrick MW. A role for myokines in muscle-bone interactions. Exerc Sport Sci Rev. 2011; 39:43–47. PMID: 21088601.
Article8. Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001; 104:531–543. PMID: 11239410.
Article9. Mera P, Laue K, Ferron M, et al. Osteocalcin signaling in myofibers Is necessary and sufficient for optimum adaptation to exercise. Cell Metab. 2016; 23:1078–1092. PMID: 27304508.
Article10. Novotny SA, Warren GL, Hamrick MW. Aging and the muscle-bone relationship. Physiology (Bethesda). 2015; 30:8–16. PMID: 25559151.
Article11. Fernández-Real JM, Vayreda M, Casamitjana R, et al. The fat-free mass compartment influences serum leptin in men. Eur J Endocrinol. 2000; 142:25–29. PMID: 10633217.12. Solberg R, Aas V, Thoresen GH, et al. Leptin expression in human primary skeletal muscle cells is reduced during differentiation. J Cell Biochem. 2005; 96:89–96. PMID: 16052473.
Article13. Wang J, Liu R, Hawkins M, et al. A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature. 1998; 393:684–688. PMID: 9641678.
Article14. Wolsk E, Mygind H, Grondahl TS, et al. Human skeletal muscle releases leptin in vivo. Cytokine. 2012; 60:667–673. PMID: 23010500.
Article15. Thomas T. The complex effects of leptin on bone metabolism through multiple pathways. Curr Opin Pharmacol. 2004; 4:295–300. PMID: 15140423.
Article16. Arounleut P, Bowser M, Upadhyay S, et al. Absence of functional leptin receptor isoforms in the POUND (Lepr(db/lb)) mouse is associated with muscle atrophy and altered myoblast proliferation and differentiation. PLoS One. 2013; 8:e72330. PMID: 23967295.
Article17. Guerra B, Santana A, Fuentes T, et al. Leptin receptors in human skeletal muscle. J Appl Physiol (1985). 2007; 102:1786–1792. PMID: 17234799.
Article18. Crespi EJ, Denver RJ. Leptin (ob gene) of the South African clawed frog Xenopus laevis. Proc Natl Acad Sci U S A. 2006; 103:10092–10097. PMID: 16782821.
Article19. Balaskó M, Soós S, Székely M, et al. Leptin and aging: Review and questions with particular emphasis on its role in the central regulation of energy balance. J Chem Neuroanat. 2014; 61-62:248–255. PMID: 25218974.
Article20. Pareja-Galeano H, Santos-Lozano A, Sanchis-Gomar F, et al. Circulating leptin and adiponectin concentrations in healthy exceptional longevity. Mech Ageing Dev. 2016; 3. 02. pii: S0047-6374(16)30019-7.
Article21. Bucci L, Yani SL, Fabbri C, et al. Circulating levels of adipokines and IGF-1 are associated with skeletal muscle strength of young and old healthy subjects. Biogerontology. 2013; 14:261–272. PMID: 23666343.
Article22. Holden KF, Lindquist K, Tylavsky FA, et al. Serum leptin level and cognition in the elderly: Findings from the Health ABC Study. Neurobiol Aging. 2009; 30:1483–1489. PMID: 18358569.
Article23. Zeki Al Hazzouri A, Stone KL, Haan MN, et al. Leptin, mild cognitive impairment, and dementia among elderly women. J Gerontol A Biol Sci Med Sci. 2013; 68:175–180. PMID: 22859388.
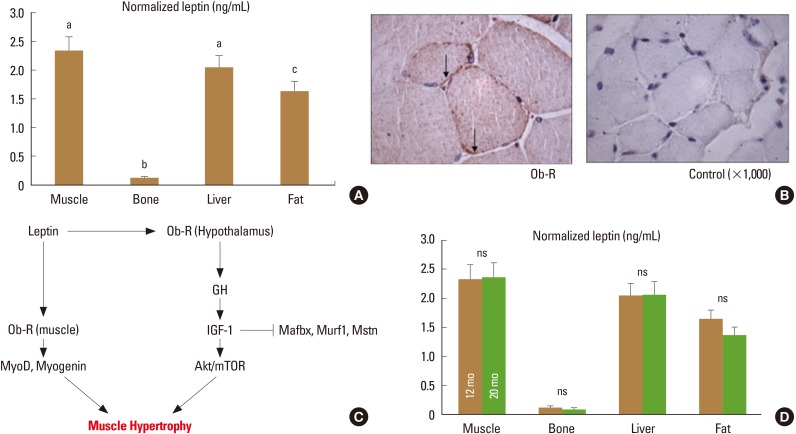
Article24. Hamrick MW, Dukes A, Arounleut P, et al. The adipokine leptin mediates muscle- and liver-derived IGF-1 in aged mice. Exp Gerontol. 2015; 70:92–96. PMID: 26220769.
Article25. Hamrick MW, McGee-Lawrence ME, Frechette DM. Fatty infiltration of skeletal muscle: mechanisms and comparisons with bone marrow adiposity. Front Endocrinol (Lausanne). 2016; 7:69. PMID: 27379021.
Article26. Chen YW, Gregory CM, Scarborough MT, et al. Transcriptional pathways associated with skeletal muscle disuse atrophy in humans. Physiol Genomics. 2007; 31:510–520. PMID: 17804603.
Article27. Sáinz N, Rodríguez A, Catalán V, et al. Leptin administration favors muscle mass accretion by decreasing FoxO3a and increasing PGC-1alpha in ob/ob mice. PLoS One. 2009; 4:e6808. PMID: 19730740.28. Bartell SM, Rayalam S, Ambati S, et al. Central (ICV) leptin injection increases bone formation, bone mineral density, muscle mass, serum IGF-1, and the expression of osteogenic genes in leptin-deficient ob/ob mice. J Bone Miner Res. 2011; 26:1710–1720. PMID: 21520275.
Article29. Anastasilakis AD, Polyzos SA, Skouvaklidou EC, et al. Circulating follistatin displays a day-night rhythm and is associated with muscle mass and circulating leptin levels in healthy, young humans. Metabolism. 2016; 65:1459–1465. PMID: 27621181.
Article30. Hamrick MW, Herberg S, Arounleut P, et al. The adipokine leptin increases skeletal muscle mass and significantly alters skeletal muscle miRNA expression profile in aged mice. Biochem Biophys Res Commun. 2010; 400:379–383. PMID: 20800581.
Article31. Cheung TH, Quach NL, Charville GW, et al. Maintenance of muscle stem-cell quiescence by microRNA-489. Nature. 2012; 482:524–528. PMID: 22358842.
Article32. Beane OS, Fonseca VC, Cooper LL, et al. Impact of aging on the regenerative properties of bone marrow-, muscle-, and adipose-derived mesenchymal stem/stromal cells. PLoS One. 2014; 9:e115963. PMID: 25541697.
Article33. Ducy P, Amling M, Takeda S, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000; 100:197–207. PMID: 10660043.34. Hamrick MW, Ding KH, Ponnala S, et al. Caloric restriction decreases cortical bone mass but spares trabecular bone in the mouse skeleton: implications for the regulation of bone mass by body weight. J Bone Miner Res. 2008; 23:870–878. PMID: 18435579.
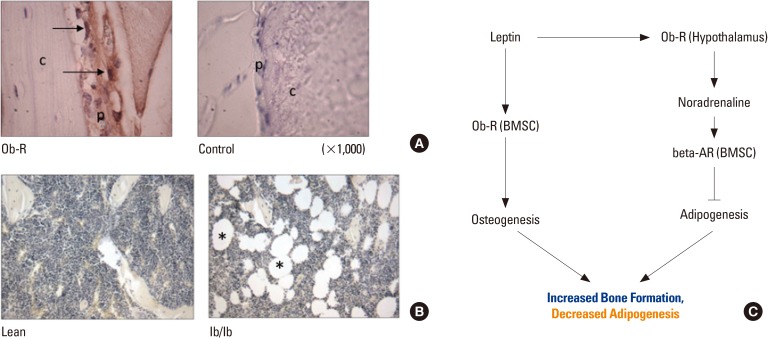
Article35. Hamrick MW, Ferrari SL. Leptin and the sympathetic connection of fat to bone. Osteoporos Int. 2008; 19:905–912. PMID: 17924050.
Article36. Hamrick MW, Della-Fera MA, Choi YH, et al. Leptin treatment induces loss of bone marrow adipocytes and increases bone formation in leptin-deficient ob/ob mice. J Bone Miner Res. 2005; 20:994–1001. PMID: 15883640.
Article37. Zhou BO, Yue R, Murphy MM, et al. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014; 15:154–168. PMID: 24953181.
Article38. Thomas T, Gori F, Khosla S, et al. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology. 1999; 140:1630–1638. PMID: 10098497.39. Yue R, Zhou BO, Shimada IS, et al. Leptin receptor promotes adipogenesis and reduces osteogenesis by regulating mesenchymal stromal cells in adult bone marrow. Cell Stem Cell. 2016; 18:782–796. PMID: 27053299.
Article40. Periyasamy-Thandavan S, Herberg S, Arounleut P, et al. Caloric restriction and the adipokine leptin alter the SDF-1 signaling axis in bone marrow and in bone marrow derived mesenchymal stem cells. Mol Cell Endocrinol. 2015; 410:64–72. PMID: 25779533.
Article41. Turner RT, Kalra SP, Wong CP, et al. Peripheral leptin regulates bone formation. J Bone Miner Res. 2013; 28:22–34. PMID: 22887758.
Article42. Hamrick MW, Della Fera MA, Choi YH, et al. Injections of leptin into rat ventromedial hypothalamus increase adipocyte apoptosis in peripheral fat and in bone marrow. Cell Tissue Res. 2007; 327:133–141. PMID: 17024416.
Article43. Lindenmaier LB, Philbrick KA, Branscum AJ, et al. Hypothalamic leptin gene therapy reduces bone marrow adiposity in ob/ob mice fed regular and high-fat diets. Front Endocrinol (Lausanne). 2016; 7:110. PMID: 27579023.
Article44. Li H, Fong C, Chen Y, et al. Beta-adrenergic signals regulate adipogenesis of mouse mesenchymal stem cells via cAMP/PKA pathway. Mol Cell Endocrinol. 2010; 323:201–207. PMID: 20363288.
Article45. Devlin MJ, Cloutier AM, Thomas NA, et al. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Miner Res. 2010; 25:2078–2088. PMID: 20229598.
Article46. Pino AM, Ríos S, Astudillo P, et al. Concentration of adipogenic and proinflammatory cytokines in the bone marrow supernatant fluid of osteoporotic women. J Bone Miner Res. 2010; 25:492–498. PMID: 19653807.
Article47. Astudillo P, Ríos S, Pastenes L, et al. Increased adipogenesis of osteoporotic human-mesenchymal stem cells (MSCs) characterizes by impaired leptin action. J Cell Biochem. 2008; 103:1054–1065. PMID: 17973271.
Article48. Hess R, Pino AM, Ríos S, et al. High affinity leptin receptors are present in human mesenchymal stem cells (MSCs) derived from control and osteoporotic donors. J Cell Biochem. 2005; 94:50–57. PMID: 15517602.
Article49. Fafián-Labora J, Fernández-Pernas P, Fuentes I, et al. Influence of age on rat bone-marrow mesenchymal stem cells potential. Sci Rep. 2015; 5:16765. PMID: 26581954.
Article50. Sui BD, Hu CH, Zheng CX, et al. Microenvironmental views on mesenchymal stem cell differentiation in aging. J Dent Res. 2016; 95:1333–1340. PMID: 27302881.
Article51. Zhang W, Ou G, Hamrick M, et al. Age-related changes in the osteogenic differentiation potential of mouse bone marrow stromal cells. J Bone Miner Res. 2008; 23:1118–1128. PMID: 18435580.
Article52. Howard JK, Flier JS. Attenuation of leptin and insulin signaling by SOCS proteins. Trends Endocrinol Metab. 2006; 17:365–371. PMID: 17010638.
Article53. Guadalupe-Grau A, Larsen S, Guerra B, et al. Influence of age on leptin induced skeletal muscle signalling. Acta Physiol (Oxf). 2014; 211:214–228. PMID: 24605926.
Article54. Hamrick MW. Leptin, bone mass, and the thrifty phenotype. J Bone Miner Res. 2004; 19:1607–1611. PMID: 15355554.
Article55. Chapman IM. Endocrinology of anorexia of ageing. Best Pract Res Clin Endocrinol Metab. 2004; 18:437–452. PMID: 15261848.
Article56. Fulgoni VL 3rd. Current protein intake in America: analysis of the National Health and Nutrition Examination Survey, 2003-2004. Am J Clin Nutr. 2008; 87:1554s–1557s. PMID: 18469286.
Article57. Wengreen HJ, Munger RG, West NA, et al. Dietary protein intake and risk of osteoporotic hip fracture in elderly residents of Utah. J Bone Miner Res. 2004; 19:537–545. PMID: 15005839.
Article58. Hubbard RE, O'Mahony MS, Calver BL, et al. Nutrition, inflammation, and leptin levels in aging and frailty. J Am Geriatr Soc. 2008; 56:279–284. PMID: 18179487.
Article59. Hamrick MW, Ding KH, Pennington C, et al. Age-related loss of muscle mass and bone strength in mice is associated with a decline in physical activity and serum leptin. Bone. 2006; 39:845–853. PMID: 16750436.
Article60. Morley JE. Anorexia, sarcopenia, and aging. Nutrition. 2001; 17:660–663. PMID: 11448592.
Article61. Demontis F, Piccirillo R, Goldberg AL, et al. The influence of skeletal muscle on systemic aging and lifespan. Aging Cell. 2013; 12:943–949. PMID: 23802635.
Article62. Gray SL, Anderson ML, Hubbard RA, et al. Frailty and incident dementia. J Gerontol A Biol Sci Med Sci. 2013; 68:1083–1090. PMID: 23419778.
Article63. Maioli S, Lodeiro M, Merino-Serrais P, et al. Alterations in brain leptin signalling in spite of unchanged CSF leptin levels in Alzheimer's disease. Aging Cell. 2015; 14:122–129. PMID: 25453257.64. Dukes A, Davis C, El Refaey M, et al. The aromatic amino acid tryptophan stimulates skeletal muscle IGF1/p70s6k/mTor signaling in vivo and the expression of myogenic genes in vitro. Nutrition. 2015; 31:1018–1024. PMID: 26059377.65. Kim H, Kim M, Kojima N, et al. Exercise and nutritional supplementation on community-dwelling elderly Japanese women with sarcopenic obesity: a randomized controlled trial. J Am Med Dir Assoc. 2016; 17:1011–1019. PMID: 27544583.
Article66. Isanejad M, Mursu J, Sirola J, et al. Association of protein intake with the change of lean mass among elderly women: The Osteoporosis Risk Factor and Prevention - Fracture Prevention Study (OSTPRE-FPS). J Nutr Sci. 2015; 4:e41. PMID: 26793306.
Article67. Welt CK, Chan JL, Bullen J, et al. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004; 351:987–997. PMID: 15342807.
Article68. Brinkoetter M, Magkos F, Vamvini M, et al. Leptin treatment reduces body fat but does not affect lean body mass or the myostatin-follistatin-activin axis in lean hypoleptinemic women. Am J Physiol Endocrinol Metab. 2011; 301:E99–E104. PMID: 21505147.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Importance of Sclerostin as Bone-Muscle Mediator Crosstalk
- Leptin-signal transduction pathways and relationship with cancer development
- Aging and Hormone Replacement Therapy
- Aging and Hormone: Estrogen, Parathyroid hormone and Bone Metabolism
- Regulation of leptin gene expression by insulin and growth hormone in mouse adipocytes