Intra-individual variability of mycophenolic acid concentration according to renal function in liver transplant recipients receiving mycophenolate monotherapy
- Affiliations
-
- 1Division of Hepatobiliary Surgery and Liver Transplantation, Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. shwang@amc.seoul.kr
- KMID: 2371562
- DOI: http://doi.org/10.14701/ahbps.2017.21.1.11
Abstract
- BACKGROUNDS/AIMS
Mycophenolate mofetil (MMF) has wide inter- and intra-individual variability of mycophenolic acid (MPA) after liver transplantation (LT). On this study, we aimed to analyse the intra-individual variability of MPA concentration in stable adult LT recipients receiving MMF monotherapy and develop a method to determine the target level in the situation of wide intra-individual variability.
METHODS
This retrospective cross-sectional study included 30 LT recipients. All patients received MMF monotherapy at a dose of 500 mg twice daily for ≥2 years and were divided into two groups based on renal function. MPA concentration-associated values were presented as mean with standard deviation and coefficient of variation (CV).
RESULTS
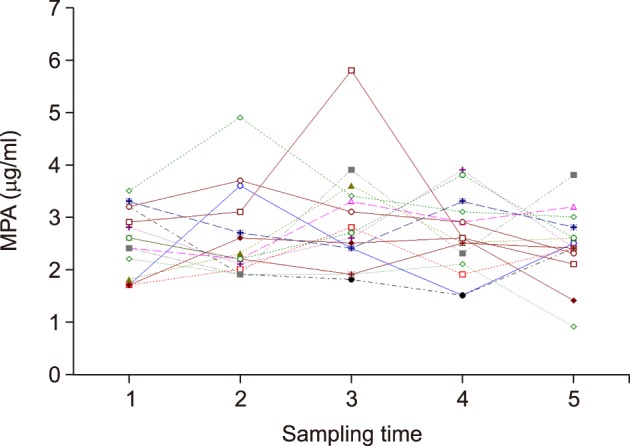
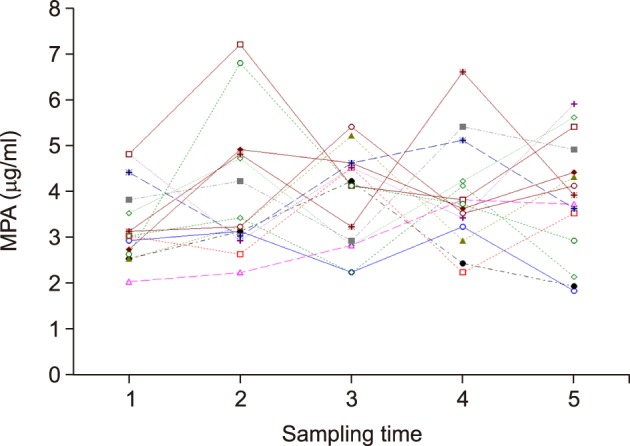
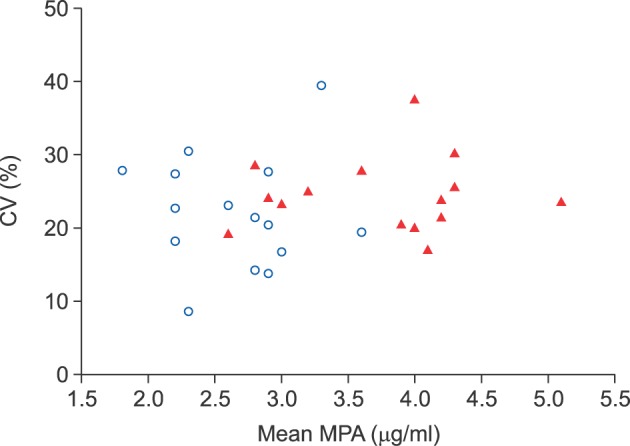
The normal renal function group (n=15) showed a mean 12-hour MPA concentration of 2.5±0.5 µg/ml (range, 1.8±0.5 to 3.6±0.7 µg/ml) and a mean CV of 20.4±7.7% (range, 8.7% to 39.4%). In the renal dysfunction group (n=15), the 12-hour MPA concentration fluctuated more widely with a mean value of 3.7±0.9 µg/ml (range, 2.8±0.8 to 5.1±1.2 µg/ml) and a mean CV of 24.5±4.9% (range, 17.1% to 37.5%). The 12-hour MPA concentration was significantly higher in the renal dysfunction group, as compared to the normal renal function group (p=0.001); whereas, the CV was not significantly different between the two groups (p=0.093).
CONCLUSIONS
We determined the inter- and intra-individual variability of 12-hour MPA concentration after LT. The results suggested that therapeutic drug monitoring of MPA is necessary due to the inter-individual and intra-individual variability of MMF pharmacokinetics, especially in LT recipients with renal dysfunction.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
Cross-sectional analysis of immunosuppressive regimens focused on everolimus after liver transplantation in a Korean high-volume transplantation center
Sang-Hyun Kang, Shin Hwang, Tae-Yong Ha, Gi-Won Song, Dong-Hwan Jung, Chul-Soo Ahn, Deok-Bog Moon, Ki-Hun Kim, Gil-Chun Park, Young-In Yoon, Yo-Han Park, Hui-Dong Cho, Jae-Hyun Kwon, Yong-Kyu Chung, Jin Uk Choi, Sung-Gyu Lee
Korean J Transplant. 2019;33(4):98-105. doi: 10.4285/jkstn.2019.33.4.98.A cross-sectional analysis of long-term immunosuppressive regimens after liver transplantation at Asan Medical Center: Increased preference for mycophenolate mofetil
Shin Hwang, Chul-Soo Ahn, Ki-Hun Kim, Deok-Bog Moon, Tae-Yong Ha, Gi-Won Song, Dong-Hwan Jung, Gil-Chun Park, Sung-Gyu Lee
Ann Hepatobiliary Pancreat Surg. 2018;22(1):19-26. doi: 10.14701/ahbps.2018.22.1.19.Pretransplant Hepatic Malignancy Increases Risk of De Novo Malignancy after Liver Transplantation
Gil-Chun Park, Shin Hwang, Chul-Soo Ahn, Ki-Hun Kim, Deok-Bog Moon, Tae-Yong Ha, Gi-Won Song, Dong-Hwan Jung, Young-In Yoon, Hui-Dong Cho, Jae-Hyun Kwon, Yong-Kyu Chung, Sang-Hyun Kang, Jin-Uk Choi, I-Ji Jung, Sung-Gyu Lee
J Korean Med Sci. 2020;35(11):. doi: 10.3346/jkms.2020.35.e69.
Reference
-
1. Jain A, Vekatramanan R, Eghtesad B, Gadomski M, Mohanka R, Marcos A, et al. Long-term outcome of adding mycophenolate mofetil to tacrolimus for nephrotoxicity following liver transplantation. Transplantation. 2005; 80:859–864. PMID: 16210976.
Article2. Jain A, Kashyap R, Dodson F, Kramer D, Hamad I, Khan A, et al. A prospective randomized trial of tacrolimus and prednisone versus tacrolimus, prednisone and mycophenolate mofetil in primary adult liver transplantation: a single center report. Transplantation. 2001; 72:1091–1097. PMID: 11579306.3. Perry I, Neuberger J. Immunosuppression: towards a logical approach in liver transplantation. Clin Exp Immunol. 2005; 139:2–10. PMID: 15606606.
Article4. Sintchak MD, Fleming MA, Futer O, Raybuck SA, Chambers SP, Caron PR, et al. Structure and mechanism of inosine monophosphate dehydrogenase in complex with the immunosuppressant mycophenolic acid. Cell. 1996; 85:921–930. PMID: 8681386.
Article5. Brunet M, Cirera I, Martorell J, Vidal E, Millán O, Jiménez O, et al. Sequential determination of pharmacokinetics and pharmacodynamics of mycophenolic acid in liver transplant patients treated with mycophenolate mofetil. Transplantation. 2006; 81:541–546. PMID: 16495801.
Article6. Hwang S, Lee SG, Ahn CS, Kim KH, Moon DB, Ha TY, et al. A clinical assessment of mycophenolate drug monitoring after liver transplantation. Clin Transplant. 2010; 24:E35–E42. PMID: 20070319.
Article7. Jain A, Venkataramanan R, Hamad IS, Zuckerman S, Zhang S, Lever J, et al. Pharmacokinetics of mycophenolic acid after mycophenolate mofetil administration in liver transplant patients treated with tacrolimus. J Clin Pharmacol. 2001; 41:268–276. PMID: 11269567.
Article8. Park YH, Hwang S, Song GW, Jung DH, Ahn CS, Kim KH, et al. Correlation between mycophenolic acid blood level and renal dysfunction in stable liver transplant recipients receiving mycophenolate monotherapy. Transplant Proc. 2014; 46:811–815. PMID: 24767354.
Article9. Borra LC, Roodnat JI, Kal JA, Mathot RA, Weimar W, van Gelder T. High within-patient variability in the clearance of tacrolimus is a risk factor for poor long-term outcome after kidney transplantation. Nephrol Dial Transplant. 2010; 25:2757–2763. PMID: 20190242.
Article10. Shuker N, van Gelder T, Hesselink DA. Intra-patient variability in tacrolimus exposure: causes, consequences for clinical management. Transplant Rev (Orlando). 2015; 29:78–84. PMID: 25687818.
Article11. Tredger JM, Brown NW, Adams J, Gonde CE, Dhawan A, Rela M, et al. Monitoring mycophenolate in liver transplant recipients: toward a therapeutic range. Liver Transpl. 2004; 10:492–502. PMID: 15048791.
Article12. Glover TE, Watson CJ, Gibbs P, Bradley JA, Ntzani EE, Kosmoliaptsis V. Conversion from calcineurin to mammalian target of rapamycin inhibitors in liver transplantation: a meta-analysis of randomized controlled trials. Transplantation. 2016; 100:621–629. PMID: 26636736.13. Hüsing A, Schmidt M, Beckebaum S, Cicinnati VR, Koch R, Thölking G, et al. Long-term renal function in liver transplant recipients after conversion from calcineurin inhibitors to mTOR inhibitors. Ann Transplant. 2015; 20:707–713. PMID: 26608590.
Article14. Bilbao I, Castells L, Rojas L, Cancino J, Dopazo C, Castro E, et al. Immunosuppression based on mycophenolate mofetil in stable liver transplanted patients. Int Immunopharmacol. 2006; 6:1977–1983. PMID: 17161351.
Article15. Orlando G, Baiocchi L, Cardillo A, Iaria G, De Liguori Carino N, De Luca L, et al. Switch to 1.5 grams MMF monotherapy for CNI-related toxicity in liver transplantation is safe and improves renal function, dyslipidemia, and hypertension. Liver Transpl. 2007; 13:46–54. PMID: 17154392.
Article16. Schmeding M, Neumann UP, Neuhaus R, Neuhaus P. Mycophenolate mofetil in liver transplantation--is monotherapy safe? Clin Transplant. 2006; 20(Suppl 17):75–79. PMID: 17100705.17. Fairbanks KD, Thuluvath PJ. Mycophenolate mofetil monotherapy in liver transplant recipients: a single center experience. Liver Transpl. 2004; 10:1189–1194. PMID: 15350013.
Article18. Reich DJ, Clavien PA, Hodge EE. MMF Renal Dysfunction after Liver Transplantation Working Group. Mycophenolate mofetil for renal dysfunction in liver transplant recipients on cyclosporine or tacrolimus: randomized, prospective, multicenter pilot study results. Transplantation. 2005; 80:18–25. PMID: 16003228.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Efficacy and Outcome of Reduced Dose of Tacrolimus in Renal Transplantation
- Pharmacokinetic Study of Mycophenolic Acid in Korean Kidney Transplant Patients
- Phased Reduction of Cyclosporine Combined with Mycophenolate Mofetil in Renal Transplant Recipients: Three-year Results of a Prospective Study
- Monitoring of Mycophenolic Acid Trough Concentration in Kidney Transplant under Cyclosporine Is Beneficial in Reducing Acute Rejection within 1 Year
- Increased Incidence of Endoscopic Erosive Esophagitis in Solid Organ Transplant Recipients