Anesth Pain Med.
2017 Jan;12(1):85-90. 10.17085/apm.2017.12.1.85.
Treatment of osteonecrosis of the femoral head by botulinum toxin type A injection to the psoas muscle: A case report
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Seoul Sungsim General Hospital, Seoul, Korea. anesthecho@naver.com
- KMID: 2369674
- DOI: http://doi.org/10.17085/apm.2017.12.1.85
Abstract
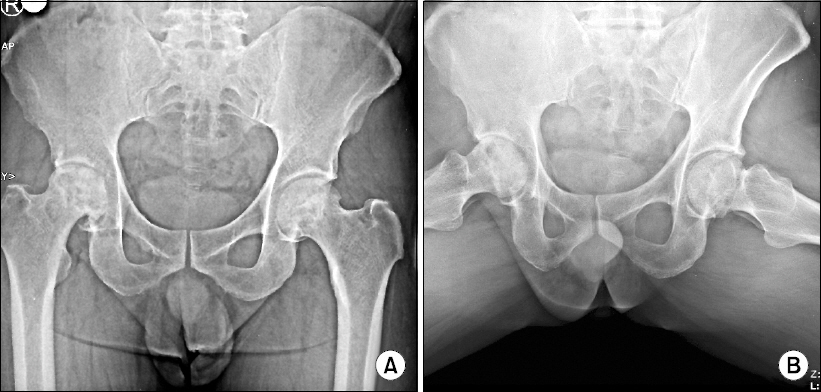
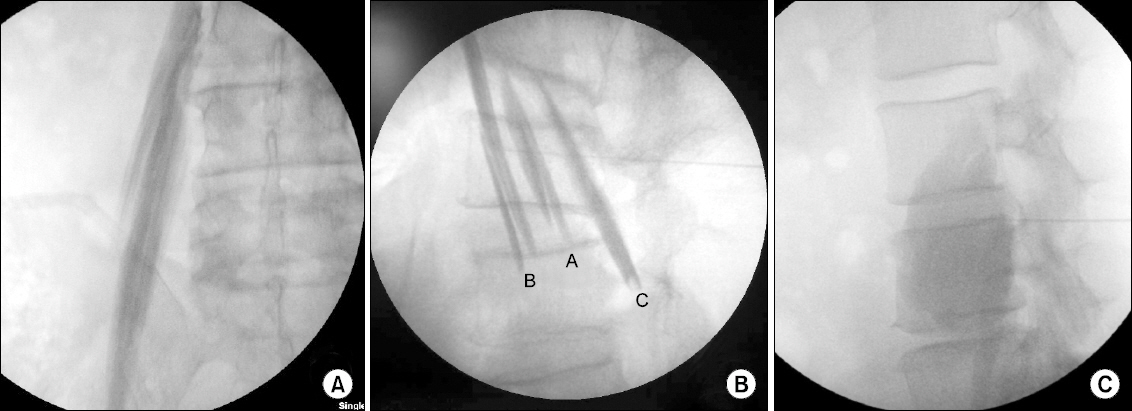
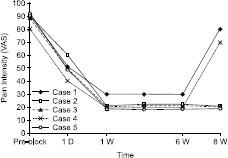
- Osteonecrosis of the femoral head (ONFH) can cause femoral head depression and cortical discontinuity. Treatment for ONFH remains challenging. We performed botulinum toxin type A injection to psoas major muscle in five patients with radiological femoral head collapse (Association Research Circulation Osseus classification stage III) who were non-responsive after two years of conservative treatment (tramadol 200 mg/day, mefenamic acid 1,000 mg/day). At two weeks after the procedure, their mean hip pain was decreased from 88 ± 0.4/100 mm to 22 ± 0.4/100 mm based on visual analogue scale (VAS). The pain was maintained at a minimum of 20/100 mm and a maximum of 30/100 mm in VAS for at least six weeks after the procedure. These values were mean ± SD. These patients were followed-up for 6 months. There was no exacerbation of pain from repeated (three times) botulinum toxin type A injection to the psoas major muscle.
MeSH Terms
Figure
Reference
-
1. Kim SY. Osteonecrosis of the femoral head. Orthopedic Surgery. 7th ed. Seok SE, editor. Seoul: Latest medical press;2013. p. 932–60.2. Mont MA, Jones LC, Hungerford DS. Nontraumatic osteonecrosis of the femoral head: ten years later. J Bone Joint Surg Am. 2006; 88:1117–32. DOI: 10.2106/00004623-200605000-00025. PMID: 16651589.3. Tripathy SK, Goyal T, Sen RK. Management of femoral head osteonecrosis: Current concepts. Indian J Orthop. 2015; 49:25–45. DOI: 10.4103/0019-5413.143911. PMID: 25593355. PMCID: PMC4292325.4. Kim JH. Orthopedic surgery for primary physician. 2th ed. Seoul: Korean medical press;2016. p. 178–80.5. Issa K, Pivec R, Kapadia BH, Banerjee S, Mont MA. Osteonecrosis of the femoral head: the total hip replacement solution. Bone Joint J. 2013; 95-B(11 Suppl A):46–50. DOI: 10.1302/0301-620X.95B11.32644. PMID: 24187351.6. Hong YC, Zhong HM, Lin T, Shi JB. Comparison of core decompression and conservative treatment for avascular necrosis of femoral head at early stage: a meta-analysis. Int J Clin Exp Med. 2015; 8:5207–16. PMID: 26131094. PMCID: PMC4483944.7. Yoshio M, Murakami G, Sato T, Sato S, Noriyasu S. The function of the psoas major muscle: passive kinetics and morphological studies using donated cadavers. J Orthop Sci. 2002; 7:199–207. DOI: 10.1007/s007760200034. PMID: 11956980.8. de Leeuw MA, Zuurmond WW, Perez RS. The psoas compartment block for hip surgery: the past, present, and future. Anesthesiol Res Pract. 2011; 2011:159541. DOI: 10.1155/2011/159541.9. Birnbaum K, Prescher A, Hessler S, Heller KD. The sensory innervation of the hip joint--an anatomical study. Surg Radiol Anat. 1997; 19:371–5. DOI: 10.1007/s00276-997-0371-5. DOI: 10.1007/BF01628504. PMID: 9479711.10. Bhave A, Zywiel MG, Ulrich SD, McGrath MS, Seyler TM, Marker DR, et al. Botulinum toxin type A injections for the management of muscle tightness following total hip arthroplasty: a case series. J Orthop Surg Res. 2009; 4:34. DOI: 10.1186/1749-799X-4-34. PMID: 19709429. PMCID: PMC2743655.11. Park GY. Botulinum Toxin A Treatment for the Improvement of Hand Function in Spastic Hemiplegia. J Korean Acad Rehabil Med. 1999; 23:240–6.12. Goldstein EM. Safety of high-dose botulinum toxin type A therapy for the treatment of pediatric spasticity. J Child Neurol. 2006; 21:189–92. PMID: 16901418.13. Lee EH, Kim SJ. Effects of electrical stimulation on the prolongation of botulinum toxin type A induced paralysis. J Korean Acad Rehabil Med. 2000; 24:1027–40.14. Touray ST, de Leeuw MA, Zuurmond WW, Perez RS. Psoas compartment block for lower extremity surgery: a meta-analysis. Br J Anaesth. 2008; 101:750–60. DOI: 10.1093/bja/aen298. PMID: 18945717.15. Moon YE, Choi JH, Park HJ, Park JH, Kim JH. Ultrasound-guided nerve block with botulinum toxin type a for intractable neuropathic pain. Toxins (Basel). 2016; 8. DOI: 10.3390/toxins8010018.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Botulinum Toxin Injection Therapy for Lingual Dystonia: A Case Report
- Improvement of Lingual Dystonia Following Cerebellar Infarction through Botulinum Toxin Injection: a Case Report
- Histopathologic Findings and Muscle Fiber Conduction Studies after Intra-muscular Injection of Botulinum Toxin in Rat
- The Complications of Botulinum Toxin Type A Chemodenervation in Strabismus
- A Case of Palatal Myoclonus Tinnitus Treated with Botolinum Toxin Injection