Diabetes Metab J.
2016 Aug;40(4):326-333. 10.4093/dmj.2016.40.4.326.
Evaluation of a Novel Glucose Area Under the Curve (AUC) Monitoring System: Comparison with the AUC by Continuous Glucose Monitoring
- Affiliations
-
- 1Department of Medicine, Shiga University of Medical Science, Otsu, Japan. sugi@belle.shiga-med.ac.jp
- 2Department of Diabetes and Endocrine Medicine, Kagoshima University Graduate School of Medical and Dental Science, Kagoshima, Japan.
- 3Central Research Laboratories, Sysmex Corporation, Kobe, Japan.
- 4Department of Clinical Laboratory, Osaka Medical Center for Cancer and Cardiovascular Diseases, Osaka, Japan.
- 5Department of Medicine, Kusatsu General Hospital, Kusatsu, Japan.
- KMID: 2368520
- DOI: http://doi.org/10.4093/dmj.2016.40.4.326
Abstract
- BACKGROUND
Management of postprandial hyperglycemia is a key aspect in diabetes treatment. We developed a novel system to measure glucose area under the curve (AUC) using minimally invasive interstitial fluid extraction technology (MIET) for simple monitoring of postprandial glucose excursions. In this study, we evaluated the relationship between our system and continuous glucose monitoring (CGM) by comparing glucose AUC obtained using MIET with that obtained using CGM for a long duration.
METHODS
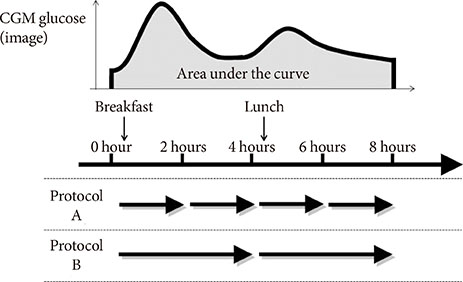
Twenty diabetic inpatients wearing a CGM system were enrolled. For MIET measurement, a plastic microneedle array was applied to the skin as pretreatment, and hydrogels were placed on the pretreated area to collect interstitial fluid. Hydrogels were replaced every 2 or 4 hours and AUC was predicted on the basis of glucose and sodium ion levels.
RESULTS
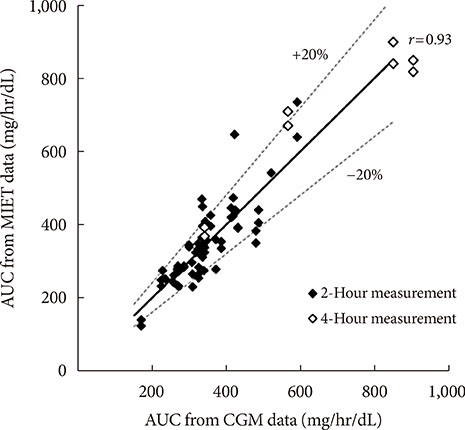
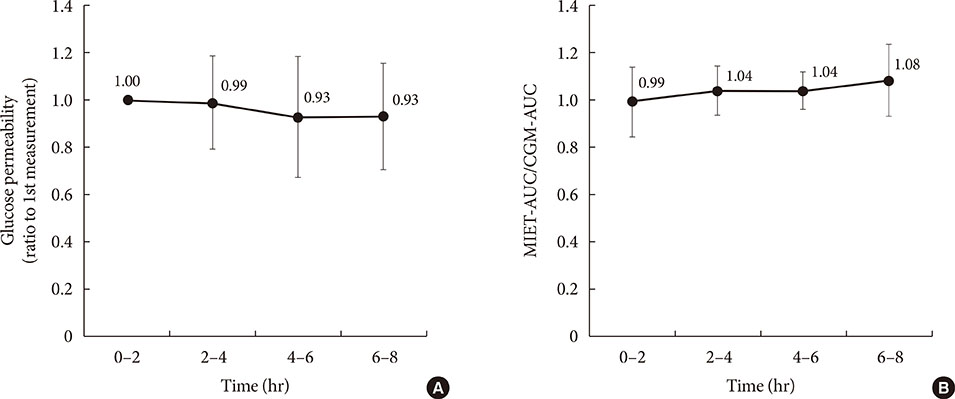
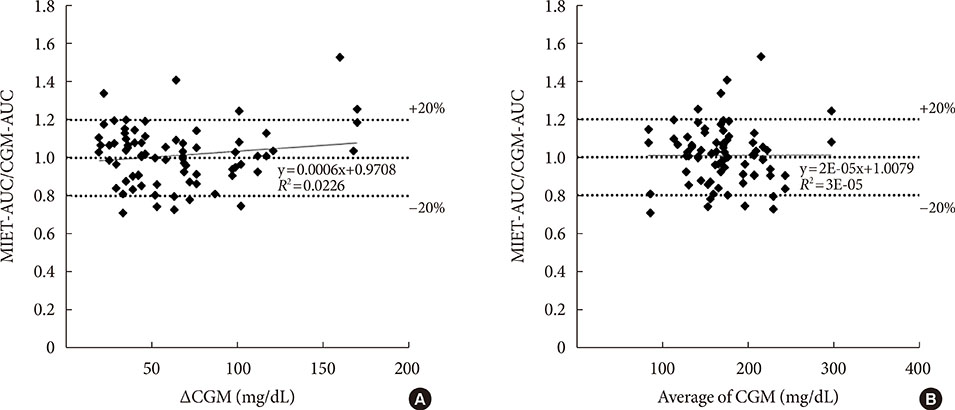
AUC predicted by MIET correlated well with that measured by CGM (r=0.93). Good performances of both consecutive 2- and 4-hour measurements were observed (measurement error: 11.7%±10.2% for 2 hours and 11.1%±7.9% for 4 hours), indicating the possibility of repetitive measurements up to 8 hours. The influence of neither glucose fluctuation nor average glucose level over the measurement accuracy was observed through 8 hours.
CONCLUSION
Our system showed good relationship with AUC values from CGM up to 8 hours, indicating that single pretreatment can cover a large portion of glucose excursion in a day. These results indicated possibility of our system to contribute to convenient monitoring of glucose excursions for a long duration.
Keyword
MeSH Terms
Figure
Reference
-
1. Ramlo-Halsted BA, Edelman SV. The natural history of type 2 diabetes: practical points to consider in developing prevention and treatment strategies. Clin Diabetes. 2000; 18:80–84.2. Hanefeld M, Chiasson JL, Koehler C, Henkel E, Schaper F, Temelkova-Kurktschiev T. Acarbose slows progression of intima-media thickness of the carotid arteries in subjects with impaired glucose tolerance. Stroke. 2004; 35:1073–1078.3. Kawamori R, Tajima N, Iwamoto Y, Kashiwagi A, Shimamoto K, Kaku K. Voglibose Ph-3 Study Group. Voglibose for prevention of type 2 diabetes mellitus: a randomised, double-blind trial in Japanese individuals with impaired glucose tolerance. Lancet. 2009; 373:1607–1614.4. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993; 329:977–986.5. Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995; 28:103–117.6. Sato T, Okada S, Hagino K, Asakura Y, Kikkawa Y, Kojima J, Watanabe T, Maekawa Y, Isobe K, Koike R, Nakajima H, Asano K. Measurement of glucose area under the curve using minimally invasive interstitial fluid extraction technology: evaluation of glucose monitoring concepts without blood sampling. Diabetes Technol Ther. 2011; 13:1194–1200.7. Sakaguchi K, Hirota Y, Hashimoto N, Ogawa W, Hamaguchi T, Matsuo T, Miyagawa J, Namba M, Sato T, Okada S, Tomita K, Matsuhisa M, Kaneto H, Kosugi K, Maegawa H, Nakajima H, Kashiwagi A. Evaluation of a minimally invasive system for measuring glucose area under the curve during oral glucose tolerance tests: usefulness of sweat monitoring for precise measurement. J Diabetes Sci Technol. 2013; 7:678–688.8. Sakaguchi K, Hirota Y, Hashimoto N, Ogawa W, Sato T, Okada S, Hagino K, Asakura Y, Kikkawa Y, Kojima J, Maekawa Y, Nakajima H. A minimally invasive system for glucose area under the curve measurement using interstitial fluid extraction technology: evaluation of the accuracy and usefulness with oral glucose tolerance tests in subjects with and without diabetes. Diabetes Technol Ther. 2012; 14:485–491.9. Sakamoto K, Kubo F, Yoshiuchi K, Ono A, Sato T, Tomita K, Sakaguchi K, Matsuhisa M, Kaneto H, Maegawa H, Nakajima H, Kashiwagi A, Kosugi K. Usefulness of a novel system for measuring glucose area under the curve while screening for glucose intolerance in outpatients. J Diabetes Investig. 2013; 4:552–559.10. Saur NM, England MR, Menzie W, Melanson AM, Trieu MQ, Berlin J, Hurley J, Krystyniak K, Kongable GL, Nasraway SA Jr. Accuracy of a novel noninvasive transdermal continuous glucose monitor in critically ill patients. J Diabetes Sci Technol. 2014; 8:945–950.11. Kuranuki S, Sato T, Okada S, Hosoya S, Seko A, Sugihara K, Nakamura T. Evaluation of postprandial glucose excursion using a novel minimally invasive glucose area-under-the-curve monitoring system. J Healthc Eng. 2013; 4:529–540.12. Watanabe T, Hagino K, Sato T. Evaluation of the effect of polymeric microneedle arrays of varying geometries in combination with a high-velocity applicator on skin permeability and irritation. Biomed Microdevices. 2014; 16:591–597.13. Ellison JM, Stegmann JM, Colner SL, Michael RH, Sharma MK, Ervin KR, Horwitz DL. Rapid changes in postprandial blood glucose produce concentration differences at finger, forearm, and thigh sampling sites. Diabetes Care. 2002; 25:961–964.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Glucose Area Under the Curve Measured Using Minimally Invasive Interstitial Fluid Extraction Technology with Continuous Glucose Monitoring System in Diabetic Patients
- Application of Continuous Glucose Monitoring System (CGMS) and Patient Education
- Comparison of Trough-Based and Area Under the Curve-Based Therapeutic Drug Monitoring of Vancomycin: An In Silico Study
- Comparison of Trough-Based and Area Under the Curve-Based Therapeutic Drug Monitoring of Vancomycin: An In Silico Study
- Glucose Management Using Continuous Glucose Monitors