J Pathol Transl Med.
2017 Jan;51(1):69-78. 10.4132/jptm.2016.10.05.
Evaluation of Pathologic Complete Response in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy: Experience in a Single Institution over a 10-Year Period
- Affiliations
-
- 1Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. eunyoon.cho@samsung.com sooyoun.cho@samsung.com
- 2Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2367682
- DOI: http://doi.org/10.4132/jptm.2016.10.05
Abstract
- BACKGROUND
Pathologic complete response (pCR) after neoadjuvant chemotherapy (NAC) has been associated with favorable clinical outcome in breast cancer patients. However, the possibility that the prognostic significance of pCR differs among various definitions has not been established.
METHODS
We retrospectively evaluated the pathologic response after NAC in 353 breast cancer patients and compared the prognoses after applying the following different definitions of pCR: ypT0/is, ypT0, ypT0/is ypN0, and ypT0 ypN0.
RESULTS
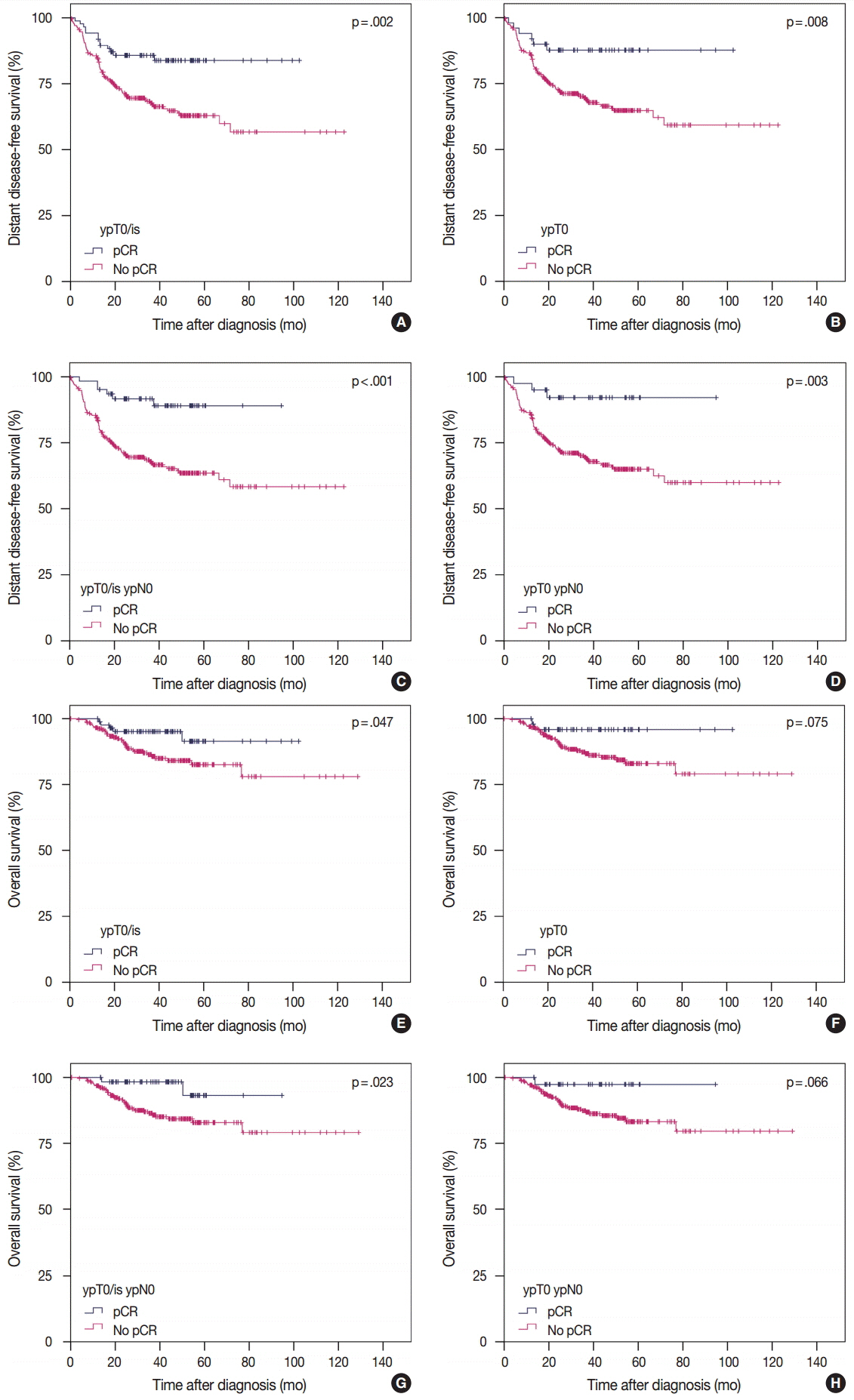
pCR was significantly associated with improved distant disease-free survival (DDFS) regardless of the definition (ypT0/is, p = .002; ypT0, p = .008; ypT0/is ypN0, p < .001; ypT0 ypN0, p = .003). Presence of tumor deposits of any size in the lymph nodes (LNs; ypN ≥ 0(i+)) was associated with worse DDFS (ypT0 ypN0 vs ypT0 ypN ≥ 0(i+), p = .036 and ypT0/is ypN0 vs ypT0/is ypN ≥ 0(i+), p = .015), and presence of isolated tumor cells was associated with decreased overall survival (OS; ypT0/is ypN0 vs ypT0/is ypN0(i+), p = .013). Residual ductal carcinoma in situ regardless of LN status showed no significant difference in DDFS or OS (DDFS: ypT0 vs ypTis, p = .373 and ypT0 ypN0 vs ypTis ypN0, p = .462; OS: ypT0 vs ypTis, p = .441 and ypT0 ypN0 vs ypTis ypN0, p = .758). In subsequent analysis using ypT0/is ypN0, pCR was associated with improved DDFS and OS in triple-negative tumors (p < .001 and p = .003, respectively).
CONCLUSIONS
Based on our study results, the prognosis and rate of pCR differ according to the definition of pCR and ypT0/is ypN0 might be considered a more preferable definition of pCR.
MeSH Terms
Figure
Cited by 1 articles
-
Wnt7a Deficiency Could Predict Worse Disease-Free and Overall Survival in Estrogen Receptor-Positive Breast Cancer
Kijong Yi, Kyueng-Whan Min, Young Chan Wi, Yeseul Kim, Su-Jin Shin, Min Sung Chung, Kiseok Jang, Seung Sam Paik
J Breast Cancer. 2017;20(4):361-367. doi: 10.4048/jbc.2017.20.4.361.
Reference
-
1. Kaufmann M, von Minckwitz G, Bear HD, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: new perspectives 2006. Ann Oncol. 2007; 18:1927–34.
Article2. Kaufmann M, von Minckwitz G, Mamounas EP, et al. Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol. 2012; 19:1508–16.
Article3. von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012; 30:1796–804.
Article4. Cortazar P, Geyer CE Jr. Pathological complete response in neoadjuvant treatment of breast cancer. Ann Surg Oncol. 2015; 22:1441–6.
Article5. Kuroi K, Toi M, Tsuda H, Kurosumi M, Akiyama F. Issues in the assessment of the pathologic effect of primary systemic therapy for breast cancer. Breast Cancer. 2006; 13:38–48.
Article6. Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001; (30):96–102.
Article7. Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998; 16:2672–85.
Article8. Robidoux A, Tang G, Rastogi P, et al. Lapatinib as a component of neoadjuvant therapy for HER2-positive operable breast cancer (NSABP protocol B-41): an open-label, randomised phase 3 trial. Lancet Oncol. 2013; 14:1183–92.
Article9. Bear HD, Tang G, Rastogi P, et al. Bevacizumab added to neoadjuvant chemotherapy for breast cancer. N Engl J Med. 2012; 366:310–20.
Article10. Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (Neo-Sphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012; 13:25–32.
Article11. Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012; 379:633–40.
Article12. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014; 384:164–72.
Article13. Corben AD, Abi-Raad R, Popa I, et al. Pathologic response and long-term follow-up in breast cancer patients treated with neoadjuvant chemotherapy: a comparison between classifications and their practical application. Arch Pathol Lab Med. 2013; 137:1074–82.
Article14. Rouzier R, Extra JM, Klijanienko J, et al. Incidence and prognostic significance of complete axillary downstaging after primary chemotherapy in breast cancer patients with T1 to T3 tumors and cytologically proven axillary metastatic lymph nodes. J Clin Oncol. 2002; 20:1304–10.
Article15. McCready DR, Hortobagyi GN, Kau SW, Smith TL, Buzdar AU, Balch CM. The prognostic significance of lymph node metastases after preoperative chemotherapy for locally advanced breast cancer. Arch Surg. 1989; 124:21–5.
Article16. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. American Joint Committee on Cancer (AJCC) cancer staging manual. 7th ed. New York: Springer;2009.17. Mazouni C, Peintinger F, Wan-Kau S, et al. Residual ductal carcinoma in situ in patients with complete eradication of invasive breast cancer after neoadjuvant chemotherapy does not adversely affect patient outcome. J Clin Oncol. 2007; 25:2650–5.
Article18. Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007; 25:4414–22.
Article19. Kurosumi M, Akiyama F, Iwase T, et al. Histopathological criteria for assessment of therapeutic response in breast cancer. Breast Cancer. 2001; 8:1–2.
Article20. Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000; 406:747–52.
Article21. Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006; 295:2492–502.
Article22. Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res. 2009; 7:4–13.
Article23. Provenzano E, Bossuyt V, Viale G, et al. Standardization of pathologic evaluation and reporting of postneoadjuvant specimens in clinical trials of breast cancer: recommendations from an international working group. Mod Pathol. 2015; 28:1185–201.
Article24. Lakhani SR, Ellis IO, Schinitt SJ, Tan PH, van de Vijver MJ. WHO classification of tumours of the breast. 4th ed. Lyon: IARC Press;2012.25. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991; 19:403–10.
Article26. Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013; 31:3997–4013.27. Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes: dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011; 22:1736–47.28. Kuroi K, Toi M, Ohno S, et al. Comparison of different definitions of pathologic complete response in operable breast cancer: a pooled analysis of three prospective neoadjuvant studies of JBCRG. Breast Cancer. 2015; 22:586–95.
Article29. Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008; 26:1275–81.
Article30. Houssami N, Macaskill P, von Minckwitz G, Marinovich ML, Mamounas E. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer. 2012; 48:3342–54.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correlation between Tumor Response to Neoadjuvant Chemotherapy and Patient Outcome in Breast Cancer
- Pathologic Findings of Residual Tumor according to the Response Rate after Neoadjuvant Chemotherapy for Breast Cancer
- Pathologic Evaluation of Breast Cancer after Neoadjuvant Therapy
- Neoadjuvant Chemotherapy with Docetaxel and Adriamycin in Breast Cancer; Clincopathologic Factors Influencing to Response Rate
- The Predictive Value of Serum HER2/neu for Response to Anthracycline-Based and Trastuzumab-Based Neoadjuvant Chemotherapy