Cancer Res Treat.
2017 Jan;49(1):10-19. 10.4143/crt.2016.058.
A Phase II Study of Poziotinib in Patients with Epidermal Growth Factor Receptor (EGFR)-Mutant Lung Adenocarcinoma Who Have Acquired Resistance to EGFR–Tyrosine Kinase Inhibitors
- Affiliations
-
- 1Center for Lung Cancer, National Cancer Center, Goyang, Korea. jymama@ncc.re.kr
- 2Department of Internal Medicine, Chungbuk National University Hospital, Cheongju, Korea.
- 3Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 4Department of Internal Medicine, Ulsan University Hospital, Ulsan, Korea.
- 5Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea.
- 6Clinical Research Team, Hanmi Pharmaceutical Co., Ltd., Seoul, Korea.
- KMID: 2367498
- DOI: http://doi.org/10.4143/crt.2016.058
Abstract
- PURPOSE
We examined the efficacy of poziotinib, a second-generation epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI) in patients with lung adenocarcinoma with activating EGFR mutations, who developed acquired resistance (AR) to EGFR-TKIs.
MATERIALS AND METHODS
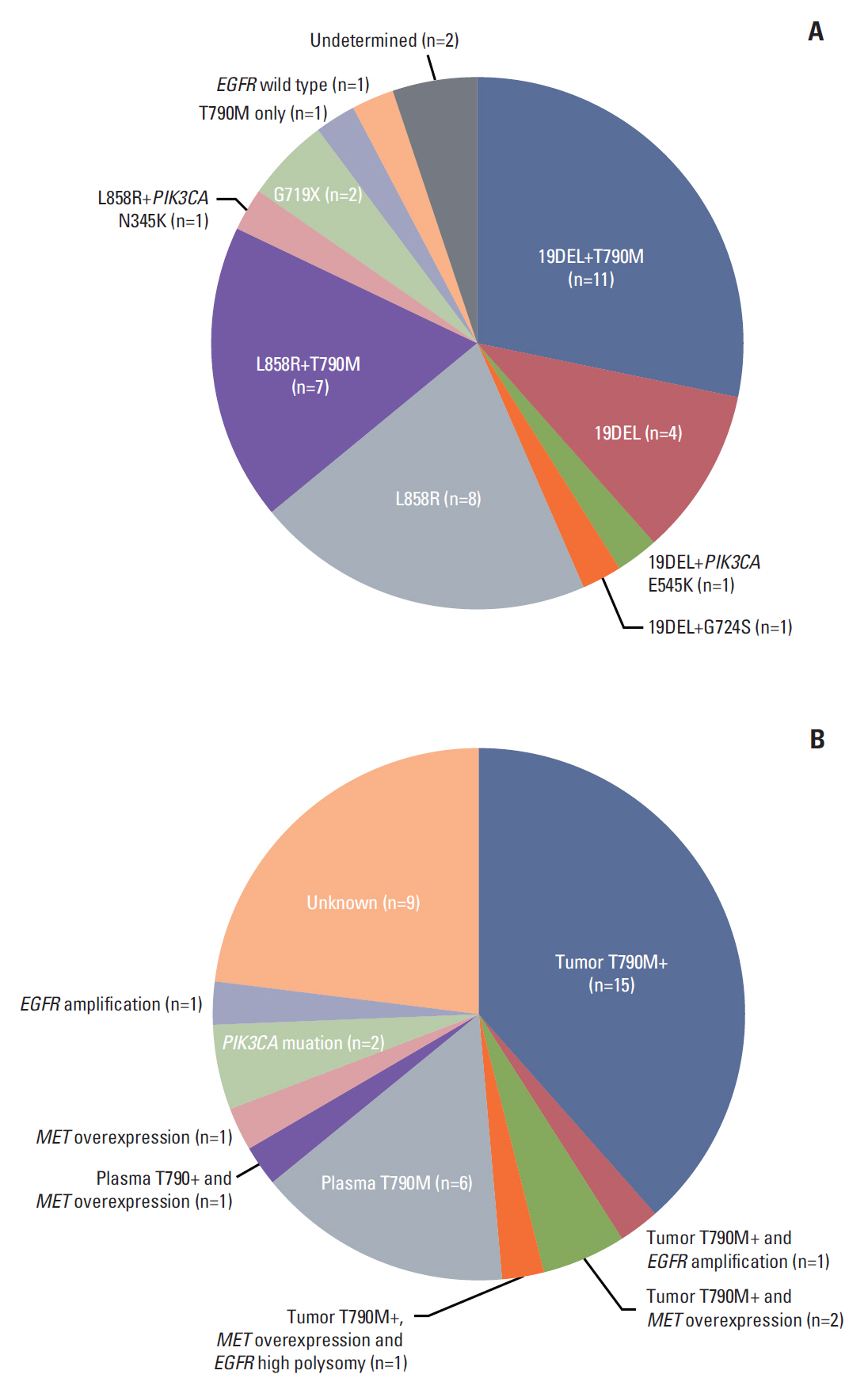
This single-arm phase II study included EGFR-mutant lung adenocarcinoma with AR to erlotinib or gefitinib based on the Jackman criteria. Patients received poziotinib 16 mg orally once daily in a 28-day cycle. The primary endpoint was progression-free survival (PFS). Prestudy tumor biopsies and blood samples were obtained to determine resistance mechanisms.
RESULTS
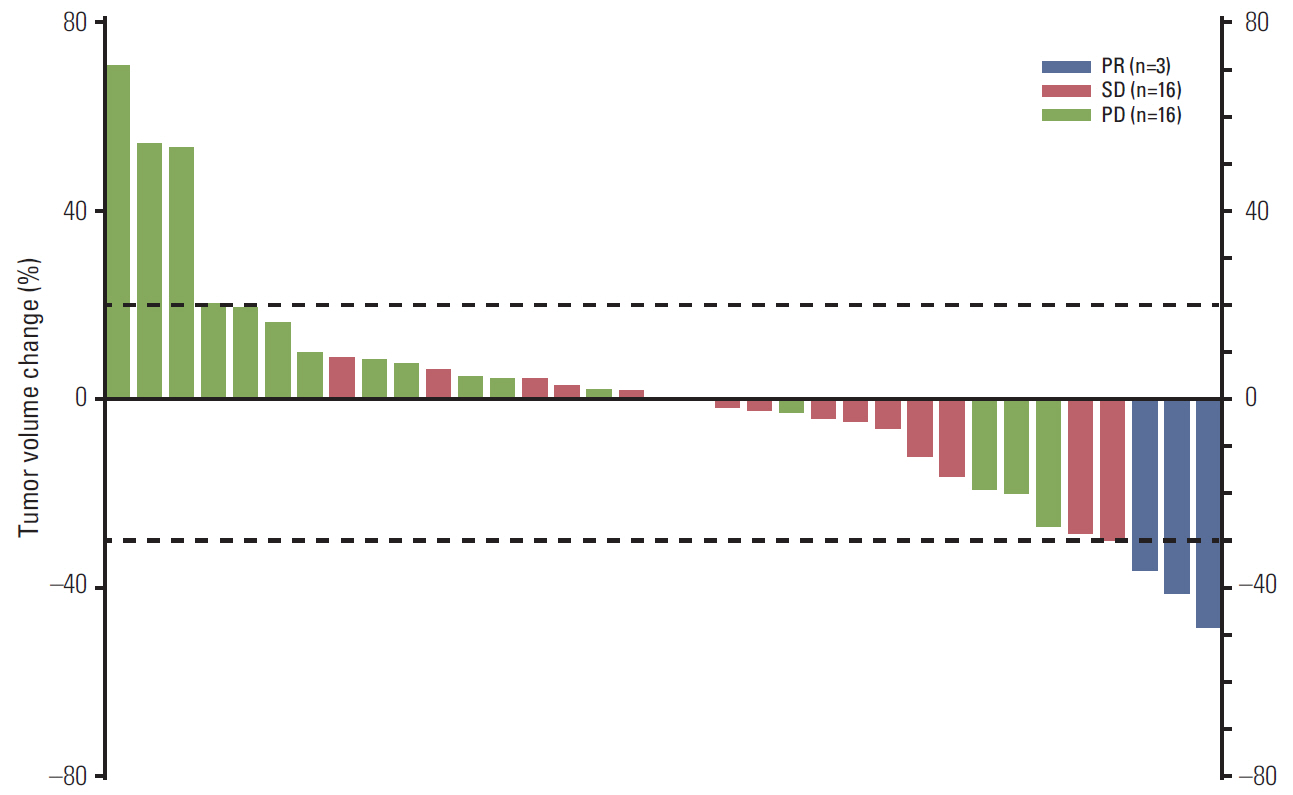
Thirty-nine patients were treated. Tumor genotyping was determined in 37 patients; 19 EGFR T790M mutations and two PIK3CA mutations were detected in the prestudy tumors, and seven T790M mutations were detected in the plasma assay. Three (8%; 95% confidence interval [CI], 2 to 21) and 17 (44%; 95% CI, 28 to 60) patients had partial response and stable disease, respectively. The median PFS and overall survival were 2.7 months (95% CI, 1.8 to 3.7) and 15.0 months (95% CI, 9.5 to not estimable), respectively. A longer PFS was observed for patients without T790M or PIK3CA mutations in tumor or plasma compared to those with these mutations (5.5 months vs. 1.8 months, p=0.003). The most frequent grade 3 adverse events were rash (59%), mucosal inflammation (26%), and stomatitis (18%). Most patients required one (n=15) or two (n=15) dose reductions.
CONCLUSION
Low activity of poziotinib was detected in patients with EGFR-mutant non-small cell lung cancer who developed AR to gefitinib or erlotinib, potentially because of severe-toxicityimposed dose limitation.
MeSH Terms
-
Adenocarcinoma*
Biopsy
Carcinoma, Non-Small-Cell Lung
Disease-Free Survival
Epidermal Growth Factor*
Erlotinib Hydrochloride
Exanthema
Humans
Inflammation
Lung
Phosphotransferases*
Plasma
Receptor, Epidermal Growth Factor*
Stomatitis
Epidermal Growth Factor
Erlotinib Hydrochloride
Phosphotransferases
Receptor, Epidermal Growth Factor
Figure
Reference
-
References
1. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010; 362:2380–8.
Article2. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011; 12:735–42.
Article3. Yang JC, Shih JY, Su WC, Hsia TC, Tsai CM, Ou SH, et al. Afatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX-Lung 2): a phase 2 trial. Lancet Oncol. 2012; 13:539–48.
Article4. Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011; 3:75ra26.
Article5. Suda K, Tomizawa K, Fujii M, Murakami H, Osada H, Maehara Y, et al. Epithelial to mesenchymal transition in an epidermal growth factor receptor-mutant lung cancer cell line with acquired resistance to erlotinib. J Thorac Oncol. 2011; 6:1152–61.
Article6. Zhang Z, Lee JC, Lin L, Olivas V, Au V, LaFramboise T, et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet. 2012; 44:852–60.
Article7. Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013; 19:2240–7.
Article8. Ohashi K, Sequist LV, Arcila ME, Moran T, Chmielecki J, Lin YL, et al. Lung cancers with acquired resistance to EGFR inhibitors occasionally harbor BRAF gene mutations but lack mutations in KRAS, NRAS, or MEK1. Proc Natl Acad Sci U S A. 2012; 109:E2127–33.
Article9. Cha MY, Lee KO, Kim M, Song JY, Lee KH, Park J, et al. Antitumor activity of HM781-36B, a highly effective pan-HER inhibitor in erlotinib-resistant NSCLC and other EGFR-dependent cancer models. Int J Cancer. 2012; 130:2445–54.
Article10. Kim YJ, Oh J, Kim TM, Han SW, Lee KW, Kim JH, et al. Phase I study to evaluate the safety and to assess the food effect of HM781-36B, a novel pan-HER inhibitor continuously given in patients with advanced solid tumors. J Clin Oncol. 2013; 31(Suppl):Abstr 2565.
Article11. Jackman D, Pao W, Riely GJ, Engelman JA, Kris MG, Janne PA, et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol. 2010; 28:357–60.
Article12. Sequist LV, Martins RG, Spigel D, Grunberg SM, Spira A, Janne PA, et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol. 2008; 26:2442–9.13. Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, Wu YL, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008; 372:1809–18.
Article14. Gridelli C, Ciardiello F, Gallo C, Feld R, Butts C, Gebbia V, et al. First-line erlotinib followed by second-line cisplatin-gemcitabine chemotherapy in advanced non-small-cell lung cancer: the TORCH randomized trial. J Clin Oncol. 2012; 30:3002–11.15. Sequist LV, Besse B, Lynch TJ, Miller VA, Wong KK, Gitlitz B, et al. Neratinib, an irreversible pan-ErbB receptor tyrosine kinase inhibitor: results of a phase II trial in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2010; 28:3076–83.
Article16. Miller VA, Hirsh V, Cadranel J, Chen YM, Park K, Kim SW, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol. 2012; 13:528–38.
Article17. Ellis PM, Shepherd FA, Millward M, Perrone F, Seymour L, Liu G, et al. Dacomitinib compared with placebo in pretreated patients with advanced or metastatic non-small-cell lung cancer (NCIC CTG BR.26): a double-blind, randomised, phase 3 trial. Lancet Oncol. 2014; 15:1379–88.
Article18. Janne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015; 372:1689–99.
Article19. Sequist LV, Soria JC, Goldman JW, Wakelee HA, Gadgeel SM, Varga A, et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2015; 372:1700–9.20. Tan CS, Cho BC, Soo RA. Next-generation epidermal growth factor receptor tyrosine kinase inhibitors in epidermal growth factor receptor-mutant non-small cell lung cancer. Lung Cancer. 2016; 93:59–68.21. Douillard JY, Ostoros G, Cobo M, Ciuleanu T, McCormack R, Webster A, et al. First-line gefitinib in Caucasian EGFR mutation-positive NSCLC patients: a phase-IV, open-label, single-arm study. Br J Cancer. 2014; 110:55–62.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Molecular Basis of Drug Resistance: Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors and Anaplastic Lymphoma Kinase Inhibitors
- Mechanisms of Acquired Resistance to Epidermal Growth Factor Receptor Inhibitors and Overcoming Strategies in Lung Cancer
- Small Cell Lung Cancer with Mutation of Epidermal Growth Factor Receptor in Patients with Lung Adenocarcinoma Resistant to Gefitinib
- Successful Rechallenge with Gefitinib for an Initial Erlotinib-Responder with Advanced Lung Adenocarcinoma
- Mechanisms of Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Resistance and Strategies to Overcome Resistance in Lung Adenocarcinoma