J Korean Med Sci.
2017 Mar;32(3):439-447. 10.3346/jkms.2017.32.3.439.
Relationship between Fractional Exhaled Nitric Oxide Level and Efficacy of Inhaled Corticosteroid in Asthma-COPD Overlap Syndrome Patients with Different Disease Severity
- Affiliations
-
- 1Department of Respiratory Medicine, Taizhou Hospital of Zhejiang Province, Linhai, China. Youzuxu@163.com
- KMID: 2366640
- DOI: http://doi.org/10.3346/jkms.2017.32.3.439
Abstract
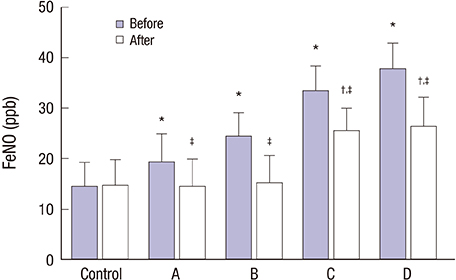
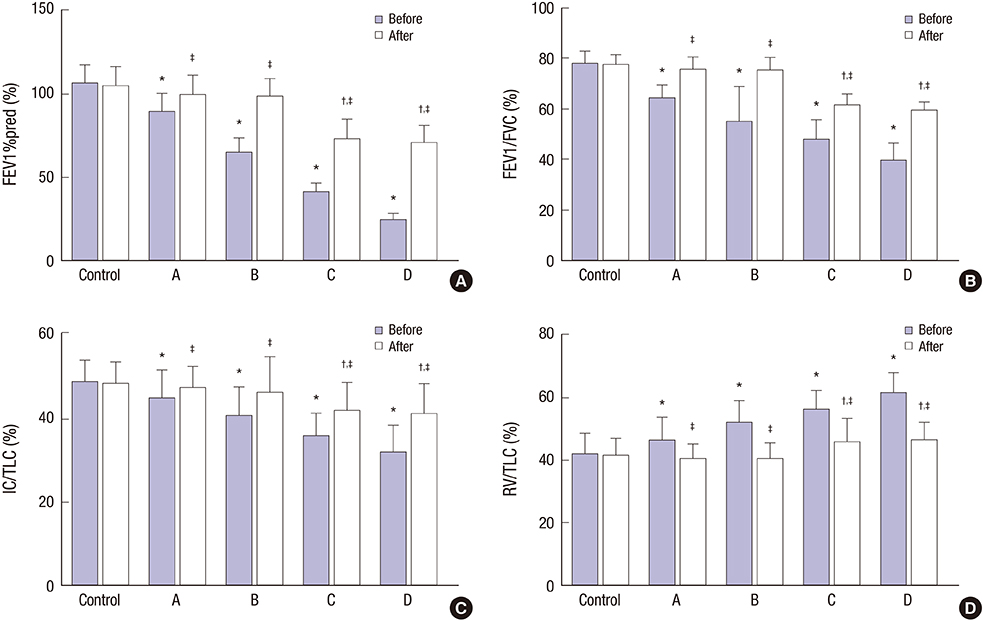
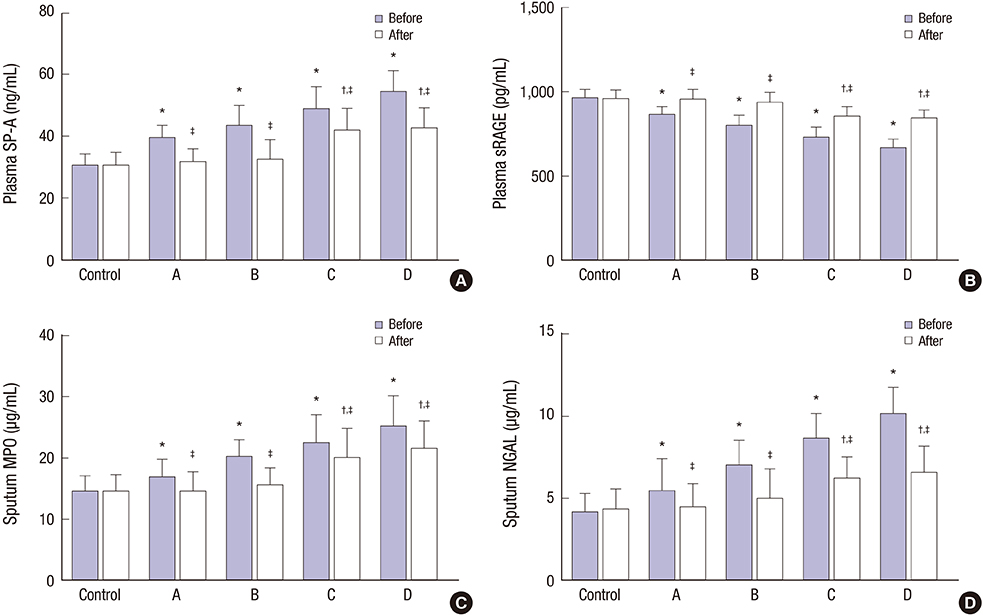
- This study explored the relationship between the fractional exhaled nitric oxide (FeNO) level and the efficacy of inhaled corticosteroid (ICS) in asthma-chronic obstructive pulmonary disease (COPD) overlap syndrome (ACOS) patients with different disease severity. A total of 127 ACOS patients with ACOS (case group) and 131 healthy people (control group) were enrolled in this study. Based on the severity of COPD, the ACOS patients were divided into: mild ACOS; moderate ACOS; severe ACOS; and extremely severe ACOS groups. We compared FeNO levels, pulmonary function parameters including percentage of forced expiratory volume in 1 second (FEV1) to predicted value (FEV1%pred), ratio of FEV1 to forced vital capacity (FEV1/FVC), inspiratory capacity to total lung capacity (IC/TLC) and residual volume to total lung capacity (RV/TLC), arterial blood gas parameters, including PH, arterial partial pressure of oxygen (PaOâ‚‚) and arterial partial pressure of carbon dioxide (PaCOâ‚‚), total serum immunoglobulin E (IgE), induced sputum eosinophil (EOS), plasma surfactant protein A (SP-A), plasma soluble receptor for advanced glycation end products (sRAGE), sputum myeloperoxidase (MPO), sputum neutrophil gelatinase-associated lipocalin (NGAL) and Asthma Control Test (ACT) scores, and COPD Assessment Test (CAT) scores. Compared with pre-treatment parameters, the FeNO levels, RV/TLC, PaCOâ‚‚, total serum IgE, induced sputum EOS, plasma SP-A, sputum MPO, sputum NGAL, and CAT scores were significantly decreased after 6 months of ICS treatment, while FEV1%pred, FEV1/FVC, IC/TLC, PH, PaOâ‚‚, plasma sRAGE, and ACT scores were significantly increased in ACOS patients with different disease severity after 6 months of ICS treatment. This finding suggests that the FeNO level may accurately predict the efficacy of ICS in the treatment of ACOS patients.
Keyword
MeSH Terms
-
Animals
Asthma
Carbon Dioxide
Cats
Eosinophils
Forced Expiratory Volume
Glycosylation End Products, Advanced
Humans
Hydrogen-Ion Concentration
Immunoglobulin E
Immunoglobulins
Inspiratory Capacity
Lipocalins
Lung Diseases, Obstructive
Neutrophils
Nitric Oxide*
Oxygen
Partial Pressure
Peroxidase
Plasma
Pulmonary Disease, Chronic Obstructive
Pulmonary Surfactant-Associated Protein A
Residual Volume
Sputum
Total Lung Capacity
Vital Capacity
Carbon Dioxide
Glycosylation End Products, Advanced
Immunoglobulin E
Immunoglobulins
Lipocalins
Nitric Oxide
Oxygen
Peroxidase
Pulmonary Surfactant-Associated Protein A
Figure
Reference
-
1. Burgel PR, Paillasseur JL, Roche N. Identification of clinical phenotypes using cluster analyses in COPD patients with multiple comorbidities. Biomed Res Int. 2014; 2014:420134.2. Zuo L, Pannell BK, Liu Z. Characterization and redox mechanism of asthma in the elderly. Oncotarget. 2016; 7:25010–25021.3. Kurashima K, Takaku Y, Ohta C, Takayanagi N, Yanagisawa T, Sugita Y. COPD assessment test and severity of airflow limitation in patients with asthma, COPD, and asthma-COPD overlap syndrome. Int J Chron Obstruct Pulmon Dis. 2016; 11:479–487.4. Papaiwannou A, Zarogoulidis P, Porpodis K, Spyratos D, Kioumis I, Pitsiou G, Pataka A, Tsakiridis K, Arikas S, Mpakas A, et al. Asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): current literature review. J Thorac Dis. 2014; 6:Suppl 1. S146–51.5. D’Urzo A, Donohue JF, Kardos P, Miravitlles M, Price D. A re-evaluation of the role of inhaled corticosteroids in the management of patients with chronic obstructive pulmonary disease. Expert Opin Pharmacother. 2015; 16:1845–1860.6. Essat M, Harnan S, Gomersall T, Tappenden P, Wong R, Pavord I, Lawson R, Everard ML. Fractional exhaled nitric oxide for the management of asthma in adults: a systematic review. Eur Respir J. 2016; 47:751–768.7. Nakamura Y, Hashiba Y, Endo J, Furuie M, Isozaki A, Yagi K. Elevated exhaled nitric oxide in anaphylaxis with respiratory symptoms. Allergol Int. 2015; 64:359–363.8. Lee JW, Shim JY, Kwon JW, Kim HY, Seo JH, Kim BJ, Kim HB, Lee SY, Jang GC, Song DJ, et al. Exhaled nitric oxide as a better diagnostic indicator for evaluating wheeze and airway hyperresponsiveness in preschool children. J Asthma. 2015; 52:1054–1059.9. Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, Olin AC, Plummer AL, Taylor DR; American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011; 184:602–615.10. Tamada T, Sugiura H, Takahashi T, Matsunaga K, Kimura K, Katsumata U, Takekoshi D, Kikuchi T, Ohta K, Ichinose M. Biomarker-based detection of asthma-COPD overlap syndrome in COPD populations. Int J Chron Obstruct Pulmon Dis. 2015; 10:2169–2176.11. Hsu JY, Wang CY, Cheng YW, Chou MC. Optimal value of fractional exhaled nitric oxide in inhaled corticosteroid treatment for patients with chronic cough of unknown cause. J Chin Med Assoc. 2013; 76:15–19.12. Harnan SE, Tappenden P, Essat M, Gomersall T, Minton J, Wong R, Pavord I, Everard M, Lawson R. Measurement of exhaled nitric oxide concentration in asthma: a systematic review and economic evaluation of NIOX MINO, NIOX VERO and NObreath. Health Technol Assess. 2015; 19:1–330.13. Mapel DW, Dalal AA, Johnson PT, Becker LK, Hunter AG. Application of the new GOLD COPD staging system to a US primary care cohort, with comparison to physician and patient impressions of severity. Int J Chron Obstruct Pulmon Dis. 2015; 10:1477–1486.14. Odler B, Ivancsó I, Somogyi V, Benke K, Tamási L, Gálffy G, Szalay B, Müller V. Vitamin D deficiency is associated with impaired disease control in asthma-COPD overlap syndrome patients. Int J Chron Obstruct Pulmon Dis. 2015; 10:2017–2025.15. Sin DD, Miravitlles M, Mannino DM, Soriano JB, Price D, Celli BR, Leung JM, Nakano Y, Park HY, Wark PA, et al. What is asthma-COPD overlap syndrome? Towards a consensus definition from a round table discussion. Eur Respir J. 2016; 48:664–673.16. American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005; 171:912–930.17. Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, Murray JJ, Pendergraft TB. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004; 113:59–65.18. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009; 34:648–654.19. Ding B, Enstone A. Asthma and chronic obstructive pulmonary disease overlap syndrome (ACOS): structured literature review and physician insights. Expert Rev Respir Med. 2016; 10:363–371.20. Zuo L, Koozechian MS, Chen LL. Characterization of reactive nitrogen species in allergic asthma. Ann Allergy Asthma Immunol. 2014; 112:18–22.21. Matsunaga K, Hirano T, Oka A, Ito K, Edakuni N. Persistently high exhaled nitric oxide and loss of lung function in controlled asthma. Allergol Int. 2016; 65:266–271.22. Brannan JD. Bronchial hyperresponsiveness in the assessment of asthma control: airway hyperresponsiveness in asthma: its measurement and clinical significance. Chest. 2010; 138:11S–7S.23. Bjermer L, Alving K, Diamant Z, Magnussen H, Pavord I, Piacentini G, Price D, Roche N, Sastre J, Thomas M, et al. Current evidence and future research needs for FeNO measurement in respiratory diseases. Respir Med. 2014; 108:830–841.24. Malinovschi A, Van Muylem A, Michiels S, Michils A. FeNO as a predictor of asthma control improvement after starting inhaled steroid treatment. Nitric Oxide. 2014; 40:110–116.25. Rouhos A, Kainu A, Piirilä P, Sarna S, Lindqvist A, Karjalainen J, Sovijärvi AR. Repeatability of exhaled nitric oxide measurements in patients with COPD. Clin Physiol Funct Imaging. 2011; 31:26–31.26. George L, Brightling CE. Eosinophilic airway inflammation: role in asthma and chronic obstructive pulmonary disease. Ther Adv Chronic Dis. 2016; 7:34–51.27. Corrao G, Arfè A, Nicotra F, Ghirardi A, Vaghi A, De Marco R, Pesci A, Merlino L, Zambon A; CRD Real-World Evidence Scientific Board. Persistence with inhaled corticosteroids reduces the risk of exacerbation among adults with asthma: a real-world investigation. Respirology. 2016; 21:1034–1040.28. Létuvé S, Druilhe A, Grandsaigne M, Aubier M, Pretolani M. Critical role of mitochondria, but not caspases, during glucocorticosteroid-induced human eosinophil apoptosis. Am J Respir Cell Mol Biol. 2002; 26:565–571.29. Fukuyama S, Matsumoto K, Kaneko Y, Kan-o K, Noda N, Tajiri-Asai Y, Nakano T, Ishii Y, Kiyohara Y, Nakanishi Y, et al. Prevalence of airflow limitation defined by pre- and post-bronchodilator spirometry in a community-based health checkup: the Hisayama Study. Tohoku J Exp Med. 2016; 238:179–184.30. Klooster K, ten Hacken NH, Hartman JE, Sciurba FC, Kerstjens HA, Slebos DJ. Determining the role of dynamic hyperinflation in patients with severe chronic obstructive pulmonary disease. Respiration. 2015; 90:306–313.31. Smargiassi A, Inchingolo R, Tagliaboschi L, Di Marco Berardino A, Valente S, Corbo GM. Ultrasonographic assessment of the diaphragm in chronic obstructive pulmonary disease patients: relationships with pulmonary function and the influence of body composition - a pilot study. Respiration. 2014; 87:364–371.32. Lee SH, Kim N, Lee CY, Ban MG, Oh YJ. Effects of dexmedetomidine on oxygenation and lung mechanics in patients with moderate chronic obstructive pulmonary disease undergoing lung cancer surgery: a randomised double-blinded trial. Eur J Anaesthesiol. 2016; 33:275–282.33. Smith DJ, Yerkovich ST, Towers MA, Carroll ML, Thomas R, Upham JW. Reduced soluble receptor for advanced glycation end-products in COPD. Eur Respir J. 2011; 37:516–522.34. Gurková E, Popelková P. Validity of asthma control test in assessing asthma control in Czech outpatient setting. Cent Eur J Public Health. 2015; 23:286–291.35. Hatipoğlu U, Subramanian A, Campbell T, Rice R, Mummadi S, Hu B, Lang DM. Intrasubject variability in total IgE levels in patients with moderate to severe persistent allergic asthma over 1 year. J Allergy Clin Immunol Pract. 2016; 4:691–696.e1.36. Iwamoto H, Gao J, Koskela J, Kinnula V, Kobayashi H, Laitinen T, Mazur W. Differences in plasma and sputum biomarkers between COPD and COPD-asthma overlap. Eur Respir J. 2014; 43:421–429.37. Choi BS, Kim KW, Lee YJ, Baek J, Park HB, Kim YH, Sohn MH, Kim KE. Exhaled nitric oxide is associated with allergic inflammation in children. J Korean Med Sci. 2011; 26:1265–1269.38. Strunk RC, Szefler SJ, Phillips BR, Zeiger RS, Chinchilli VM, Larsen G, Hodgdon K, Morgan W, Sorkness CA, Lemanske RF Jr, et al. Relationship of exhaled nitric oxide to clinical and inflammatory markers of persistent asthma in children. J Allergy Clin Immunol. 2003; 112:883–892.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Measurement and Interpretation of Fractional Exhaled Nitric Oxide
- Utility of Fractional Exhaled Nitric Oxide in the Diagnosis of Asthma and the Assessment of Asthma Control
- Measurements of fractional exhaled nitric oxide in pediatric asthma
- Clinical application of fractional exhaled nitric oxide in pediatric allergic rhinitis
- Lack of Association between Inhaled Corticosteroid Use Based on the Exhaled Nitric Oxide and Acute Exacerbation of Chronic Obstructive Pulmonary Disease