Korean J Ophthalmol.
2015 Oct;29(5):294-300. 10.3341/kjo.2015.29.5.294.
Long-term Surgical Outcomes of the Multi-purpose Conical Porous Synthetic Orbital Implant
- Affiliations
-
- 1Department of Ophthalmology and Visual Science, Seoul St. Mary's Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea. yswoph@catholic.ac.kr
- 2Department of Ophthalmology, Bucheon St. Mary's Hospital, The Catholic University of Korea College of Medicine, Bucheon, Korea.
- 3Department of Ophthalmology, Daejeon St. Mary's Hospital, The Catholic University of Korea College of Medicine, Daejeon, Korea.
- KMID: 2363802
- DOI: http://doi.org/10.3341/kjo.2015.29.5.294
Abstract
- PURPOSE
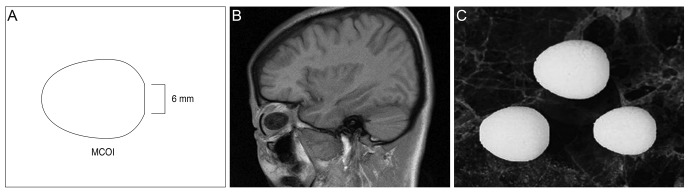
We present clinical results of the use of the multipurpose conical porous synthetic orbital implant (MCOI) in surgical procedures of evisceration, enucleation, and secondary enucleation in ophthalmology patients.
METHODS
A retrospective review was performed of 59 eyes in which conical implants were used, including 36 cases of eviscerations, 11 enucleations, and 9 secondary enucleations. In all of the cases, the follow-up period was greater than six months between 2004 and 2013. The results focus on documenting surgical findings, as well as postoperative complications among patients.
RESULTS
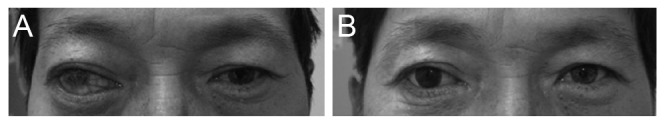
Superior sulcus deformities were found in six eyes (10.2% of conical implant patients), and two eyes received additional surgical interventions to correct the deformities (3.4%). Blepharoptosis was found in four eyes (6.8%), two of which received upper eyelid blepharoplasty (3.4%). Fornix shortening was reported in only one eye (1.7%). Forty-one eyes had a satisfactory cosmetic appearance after the final prosthetic fitting of conical implants (69.5%). The most frequent postoperative complication was orbital implant exposure, which seemed to occur when the preoperative status of the conjunctiva, Tenon's capsule, and sclera preservation were poor in the eyes of the patients.
CONCLUSIONS
There was a lower incidence of blepharoptosis and fornix shortening with the MCOI in comparison to spherical implants, while the incidence of orbital implant exposure was similar with the MCOI in comparison to other types of orbital implants. In addition, the MCOI may have advantages with respect to postoperative cosmetic outcomes.
MeSH Terms
Figure
Reference
-
1. Smit TJ, Koornneef L, Zonneveld FW, et al. Computed tomography in the assessment of the postenucleation socket syndrome. Ophthalmology. 1990; 97:1347–1351. PMID: 2243686.
Article2. Marshak H, Dresner SC. Multipurpose conical orbital implant in evisceration. Ophthal Plast Reconstr Surg. 2005; 21:376–378.
Article3. Hornblass A, Biesman BS, Eviatar JA. Current techniques of enucleation: a survey of 5,439 intraorbital implants and a review of the literature. Ophthal Plast Reconstr Surg. 1995; 11:77–86.4. Lee JH, Cho WC. A case of Medpor orbital implant infection. J Korean Ophthalmol Soc. 2001; 42:783–787.5. Rubin PA, Popham J, Rumelt S, et al. Enhancement of the cosmetic and functional outcome of enucleation with the conical orbital implant. Ophthalmology. 1998; 105:919–925. PMID: 9593398.
Article6. Chalasani R, Poole-Warren L, Conway RM, Ben-Nissan B. Porous orbital implants in enucleation: a systematic review. Surv Ophthalmol. 2007; 52:145–155. PMID: 17355854.
Article7. Custer PL. Enucleation: past, present, and future. Ophthal Plast Reconstr Surg. 2000; 16:316–321.
Article8. Jordan DR, Gilberg S, Bawazeer A. Coralline hydroxyapatite orbital implant (bio-eye): experience with 158 patients. Ophthal Plast Reconstr Surg. 2004; 20:69–74.
Article9. Blaydon SM, Shepler TR, Neuhaus RW, et al. The porous polyethylene (Medpor) spherical orbital implant: a retrospective study of 136 cases. Ophthal Plast Reconstr Surg. 2003; 19:364–371.10. Colen TP, Paridaens DA, Lemij HG, et al. Comparison of artificial eye amplitudes with acrylic and hydroxyapatite spherical enucleation implants. Ophthalmology. 2000; 107:1889–1894. PMID: 11013194.
Article11. Kronish JW, Gonnering RS, Dortzbach RK, et al. The pathophysiology of the anophthalmic socket. Part II. Analysis of orbital fat. Ophthal Plast Reconstr Surg. 1990; 6:88–95.
Article12. Kaltreider SA, Jacobs JL, Hughes MO. Predicting the ideal implant size before enucleation. Ophthal Plast Reconstr Surg. 1999; 15:37–43.
Article13. Alwitry A, West S, King J, et al. Long-term follow-up of porous polyethylene spherical implants after enucleation and evisceration. Ophthal Plast Reconstr Surg. 2007; 23:11–15.
Article14. Jung SK, Cho WK, Paik JS, Yang SW. Long-term surgical outcomes of porous polyethylene orbital implants: a review of 314 cases. Br J Ophthalmol. 2012; 96:494–498. PMID: 22096144.
Article15. Lee S, Maronian N, Most SP, et al. Porous high-density polyethylene for orbital reconstruction. Arch Otolaryngol Head Neck Surg. 2005; 131:446–450. PMID: 15897425.
Article16. Klawitter JJ, Bagwell JG, Weinstein AM, Sauer BW. An evaluation of bone growth into porous high density polyethylene. J Biomed Mater Res. 1976; 10:311–323. PMID: 1254618.
Article17. Spector M, Flemming WR, Sauer BW. Early tissue infiltrate in porous polyethylene implants into bone: a scanning electron microscope study. J Biomed Mater Res. 1975; 9:537–542. PMID: 1176523.
Article18. Baek SH. Clinical effect of porous polyethylene (Medpor(r)) orbital implant. J Korean Ophthalmol Soc. 2000; 41:1858–1863.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of the Synthetic Bone Glass on the Fibrovascularization into Porous Polyethylene Orbital Implant
- Scleral Eversion Technique for Porous Polyethylene Orbital Implant after Evisceration
- A Case of Orbital Abscess following Porous Orbital Implant Infection
- Reconstruction of extended orbital floor fracture using an implantation method of gamma-shaped porous polyethylene
- Clinical Effect of Porous Polyethylene (Medpor(r))Orbital Implant