Korean J Ophthalmol.
2015 Jun;29(3):190-194. 10.3341/kjo.2015.29.3.190.
Assessment of Patient Pain Experience during Intravitreal 27-Gauge Bevacizumab and 30-Gauge Ranibizumab Injection
- Affiliations
-
- 1Department of Ophthalmology, Adiyaman University School of Medicine, Adiyaman, Turkey. meteglr@yahoo.com
- 2Department of Ophthalmology, Adiyaman University Education and Research Hospital, Adiyaman, Turkey.
- KMID: 2363758
- DOI: http://doi.org/10.3341/kjo.2015.29.3.190
Abstract
- PURPOSE
To compare pain scores of patients during intravitreal 27-gauge bevacizumab and 30-gauge ranibizumab injection procedures.
METHODS
Seventy eyes of 70 patients who had not previously undergone intravitreal anti-vascular endothelial growth factor therapy were included in this study. Thirty-five patients received ranibizumab and 35 patients received bevacizumab. The diagnoses of the patients were: 27 age related macular degeneration, 15 diabetic macular edema, 9 diabetic vitreous hemorrhage, 6 central retinal vein occlusion, 11 branch retinal vein occlusion and 2 central serous chorioretinopathy. Bevacizumab (1.25 mg/0.05 mL) was injected into the vitreous cavity using a 27-gauge needle, and ranibizumab (0.5 mg/0.05 mL) was injected with 30-gauge needle. Patients were asked just after the injection to rate their perceived pain during the injection using the visual analogue scale (VAS) of 0 (no pain) to 10 (unbearable/worst pain). The average of these scores was used as the primary outcome.
RESULTS
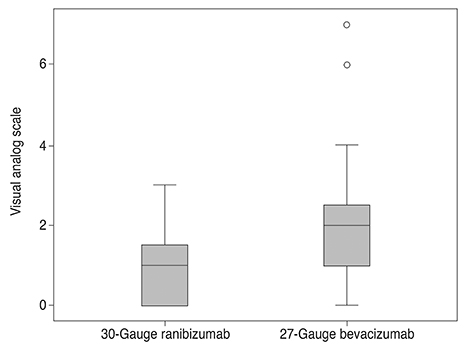
The VAS pain scores in the ranibizumab and bevacizumab groups were 1.06 +/- 0.91 (range, 0 to 3) and 1.94 +/- 1.55 (range, 0 to 7), respectively, a significant difference (p = 0.005). Patients <65 and > or =65 years of age in both the ranibizumab and bevacizumab groups were then compared. For patients <65, there was a significant difference in the average VAS pain scores between groups (p = 0.003). However, for patients > or =65 years, there was not a significant difference in the average VAS pain scores between groups (p = 0.238). Female and male patients in both ranibizumab and bevacizumab groups were also compared. For female patients, there was a significant difference in the average VAS pain scores between groups (p = 0.016), although not for male patients (p = 0.078).
CONCLUSIONS
Thirty-gauge intravitreal injection is more comfortable than 27-gauge injection. Injection of bevacizumab with 30-gauge needle syringes may be more tolerable for patients.
MeSH Terms
-
Aged
Aged, 80 and over
Angiogenesis Inhibitors/*administration & dosage
Antibodies, Monoclonal, Humanized/*administration & dosage
Bevacizumab/*administration & dosage
Diabetic Retinopathy/drug therapy/physiopathology
Female
Humans
*Intravitreal Injections
Macular Degeneration/drug therapy/physiopathology
Macular Edema/drug therapy/physiopathology
Male
Middle Aged
Pain Measurement
Ranibizumab/*administration & dosage
Retinal Vein Occlusion/drug therapy/physiopathology
Angiogenesis Inhibitors
Antibodies, Monoclonal, Humanized
Bevacizumab
Ranibizumab
Figure
Reference
-
1. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–1431.2. Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006; 113:1695.3. Pieramici DJ, Rabena M, Castellarin AA, et al. Ranibizumab for the treatment of macular edema associated with perfused central retinal vein occlusions. Ophthalmology. 2008; 115:e47–e54.4. Park SC, Su D, Tello C. Anti-VEGF therapy for the treatment of glaucoma: a focus on ranibizumab and bevacizumab. Expert Opin Biol Ther. 2012; 12:1641–1647.5. Peyman GA, Lad EM, Moshfeghi DM. Intravitreal injection of therapeutic agents. Retina. 2009; 29:875–912.6. Tewari A, Shah GK, Dhalla MS, Blinder KJ. Surface anesthesia for office-based retinal procedures. Retina. 2007; 27:804–805.7. Green-Simms AE, Ekdawi NS, Bakri SJ. Survey of intravitreal injection techniques among retinal specialists in the United States. Am J Ophthalmol. 2011; 151:329–332.8. Moisseiev E, Regenbogen M, Bartfeld Y, Barak A. Evaluation of pain in intravitreal bevacizumab injections. Curr Eye Res. 2012; 37:813–817.9. Rifkin L, Schaal S. Factors affecting patients' pain intensity during in office intravitreal injection procedure. Retina. 2012; 32:696–700.10. Reed MD, Van Nostran W. Assessing pain intensity with the visual analog scale: a plea for uniformity. J Clin Pharmacol. 2014; 54:241–244.11. Aslankurt M, Aslan L, Baskan AM, et al. Pain and cooperation in patients having dominant-side or nondominant-side phacoemulsification. J Cataract Refract Surg. 2014; 40:199–202.12. Mirshahi A, Lashay A, Roozbahani M, et al. Pain score of patients undergoing single spot, short pulse laser versus conventional laser for diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2013; 251:1103–1107.13. Chen D, Lian Y, Li J, et al. Monitor corneal epithelial healing under bandage contact lens using ultrahigh-resolution optical coherence tomography after pterygium surgery. Eye Contact Lens. 2014; 40:175–180.14. Narvaez J, Wessels I, Bacon G, et al. Prospective randomized evaluation of short-term complications when using buffered or unbuffered lidocaine 1% with epinephrine for blepharoplasty surgery. Ophthal Plast Reconstr Surg. 2010; 26:33–35.15. Knecht PB, Michels S, Sturm V, et al. Tunnelled versus straight intravitreal injection: intraocular pressure changes, vitreous reflux, and patient discomfort. Retina. 2009; 29:1175–1181.16. Rodrigues EB, Grumann A Jr, Penha FM, et al. Effect of needle type and injection technique on pain level and vitreal reflux in intravitreal injection. J Ocul Pharmacol Ther. 2011; 27:197–203.17. Eaton AM, Gordon GM, Wafapoor H, et al. Assessment of novel guarded needle to increase patient comfort and decrease injection time during intravitreal injection. Ophthalmic Surg Lasers Imaging Retina. 2013; 44:561–568.18. Pulido JS, Zobitz ME, An KN. Scleral penetration force requirements for commonly used intravitreal needles. Eye (Lond). 2007; 21:1210–1211.19. Levin LA, Nilsson SF, Hoeve JV, Wu S. Adler's physiology of the eye. In : Levin LA, Nilsson SF, Hoeve JV, Wu S, editors. Sensory innervation of the eye. 11th ed. London: Elsevier;2011. p. 363–384.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ocular Pain According to Needle Diameter during Intravitreal Injection
- Electron Microscopy of Needle Tips Following Intravitreal Injections Using 30-Gauge Needles
- Comparison of Intravitreal Bevacizumab and Ranibizumab Injections in Aggressive and Type 1 Retinopathy of Prematurity
- Effect of 23-gauge Sutureless Vitrectomy & Preoperative Bevacizumab on Results of Diabetic Vitrectomy
- Relationship between Pain and Injection Site during Intravitreal Injection