Lab Anim Res.
2016 Dec;32(4):200-207. 10.5625/lar.2016.32.4.200.
Diallyl disulfide attenuates acetaminophen-induced renal injury in rats
- Affiliations
-
- 1Ministry of Food and Drug Safety, Cheongju, Korea.
- 2College of Veterinary Medicine, Chonnam National University, Gwangju, Korea. toxkim@jnu.ac.kr
- 3Jeonbuk Department of Inhalation Research, Korea Institute of Toxicology, Jeongeup, Korea.
- KMID: 2362911
- DOI: http://doi.org/10.5625/lar.2016.32.4.200
Abstract
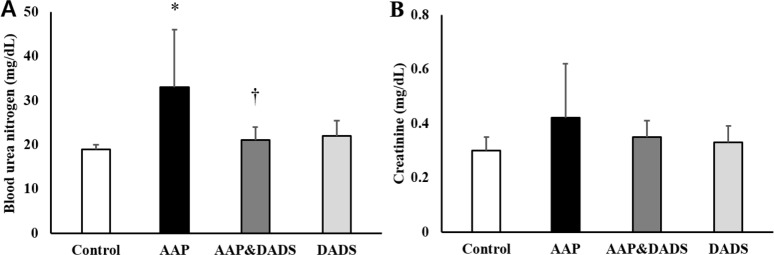
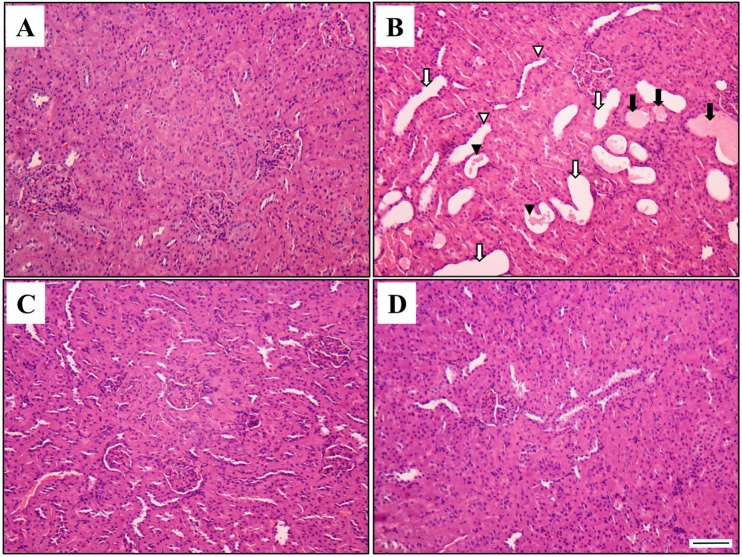
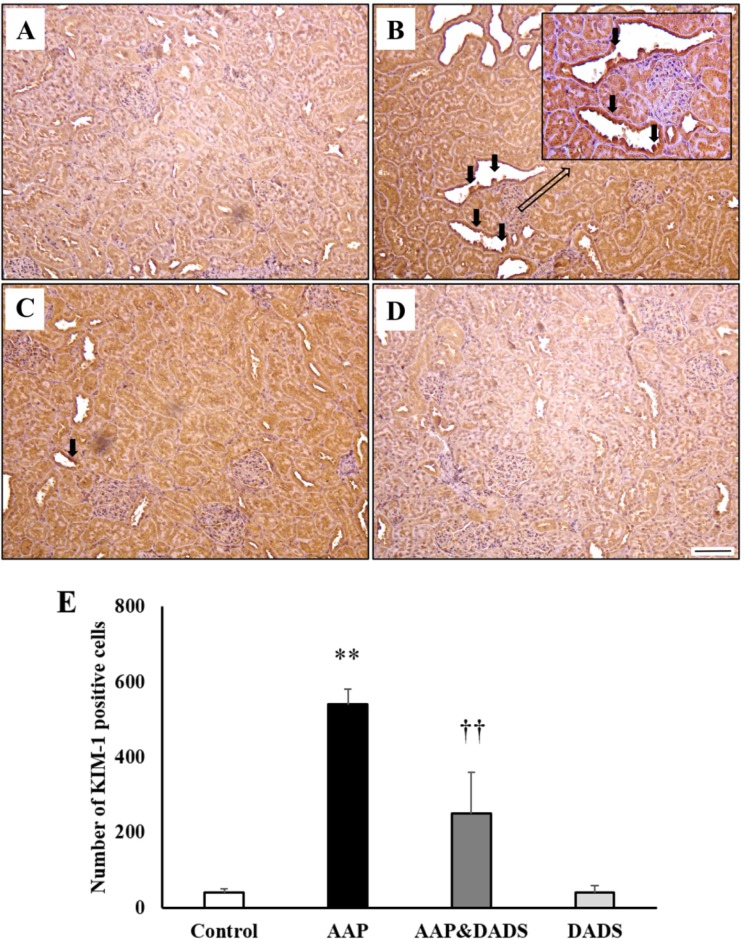
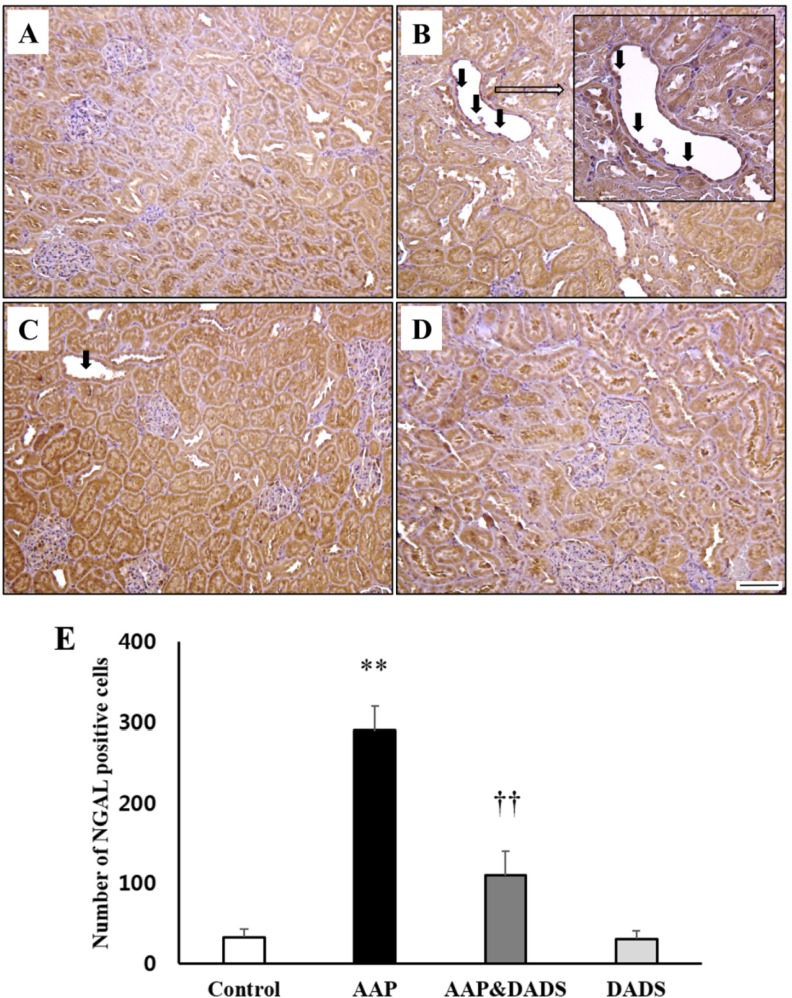
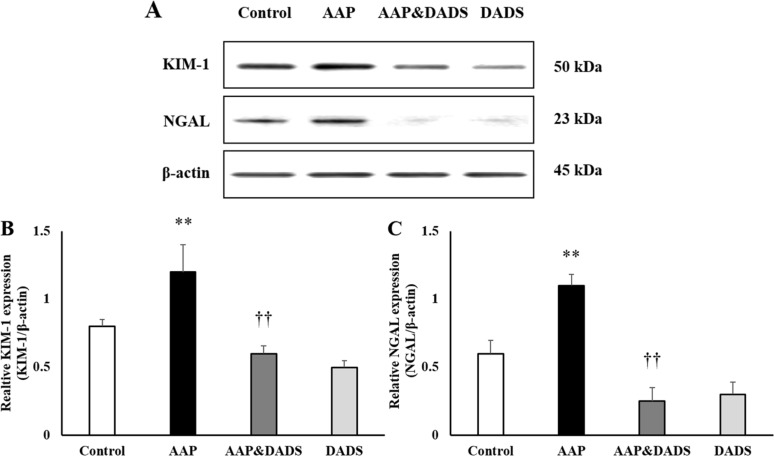
- This study investigated the protective effects of diallyl disulfide (DADS) against acetaminophen (AAP)-induced acute renal injury in male rats. We also investigated the effects of DADS on kidney injury molecule-1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL), which are novel biomarkers of nephrotoxicity in renal tissues, in response to AAP treatment. The following four experimental groups were evaluated: (1) vehicle control, (2) AAP (1,000 mg/kg), (3) AAP&DADS, and (4) DADS (50 mg/kg/day). AAP treatment caused acute kidney injury evidenced by increased serum blood urea nitrogen (BUN) levels and histopathological alterations. Additionally, Western blot and immunohistochemistry analysis showed increased expression of KIM-1 and NGAL proteins in renal tissues of AAP-treated rats. In contrast, DADS pretreatment significantly attenuated the AAP-induced nephrotoxic effects, including serum BUN level and expression of KIM-1 and NGAL proteins. Histopathological studies confirmed the renoprotective effect of DADS. The results suggest that DADS prevents AAP-induced acute nephrotoxicity, and that KIM-1 and NGAL may be useful biomarkers for the detection and monitoring of acute kidney injury associated with AAP exposure.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Exploring the role and mechanisms of diallyl trisulfide and diallyl disulfide in chronic constriction-induced neuropathic pain in rats
Gang Wang, Yan Yang, Chunfeng Wang, Jianzhong Huang, Xiao Wang, Ying Liu, Hao Wang
Korean J Pain. 2020;33(3):216-225. doi: 10.3344/kjp.2020.33.3.216.
Reference
-
1. Kanno S, Tomizawa A, Hiura T, Osanai Y, Kakuta M, Kitajima Y, Koiwai K, Ohtake T, Ujibe M, Ishikawa M. Melatonin protects on toxicity by acetaminophen but not on pharmacological effects in mice. Biol Pharm Bull. 2006; 29(3):472–476. PMID: 16508148.
Article2. Hengy B, Hayi-Slayman D, Page M, Christin F, Baillon JJ, Ber CE, Allaouchiche B, Rimmele T. Acute renal failure after acetaminophen poisoning: report of three cases. Can J Anaesth. 2009; 56(10):770–774. PMID: 19639374.3. Ghosh J, Das J, Manna P, Sil PC. Acetaminophen induced renal injury via oxidative stress and TNF-alpha production: therapeutic potential of arjunolic acid. Toxicology. 2010; 268(1-2):8–18. PMID: 19922764.4. Singh VP, Singh N, Jaggi AS. A review on renal toxicity profile of common abusive drugs. Korean J Physiol Pharmacol. 2013; 17(4):347–357. PMID: 23946695.
Article5. Schnellman RG. Toxic responses of the kidney. In : Klassen CD, editor. Casarett & Doull's Toxicology: The Basic Science of Poisons. 6th ed. New York: McGraw-Hill Medical Publishing Division;2001. p. 491–514.6. Mediæ B, Rovcanin B, Vujovic KS, Obradovic D, Duric D, Prostran M. Evaluation of Novel Biomarkers of Acute Kidney Injury: The Possibilities and Limitations. Curr Med Chem. 2016; 23(19):1981–1997. PMID: 26860999.7. Schrezenmeier EV, Barasch J, Budde K, Westhoff T, Schmidt-Ott KM. Biomarkers in acute kidney injury - pathophysiological basis and clinical performance. Acta Physiol (Oxf). 2016; 30:12764.
Article8. Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2004; 286(3):F552–F563. PMID: 14600030.
Article9. Huo W, Zhang K, Nie Z, Li Q, Jin F. Kidney injury molecule-1 (KIM-1): a novel kidney-specific injury molecule playing potential double-edged functions in kidney injury. Transplant Rev (Orlando). 2010; 24(3):143–146. PMID: 20447817.
Article10. Bolignano D, Donato V, Coppolino G, Campo S, Buemi A, Lacquaniti A, Buemi M. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am J Kidney Dis. 2008; 52(3):595–605. PMID: 18725016.
Article11. Mishra J, Ma Q, Kelly C, Mitsnefes M, Mori K, Barasch J, Devarajan P. Kidney NGAL is a novel early marker of acute injury following transplantation. Pediatr Nephrol. 2006; 21(6):856–863. PMID: 16528543.
Article12. Soni SS, Cruz D, Bobek I, Chionh CY, Nalesso F, Lentini P, de Cal M, Corradi V, Virzi G, Ronco C. NGAL: a biomarker of acute kidney injury and other systemic conditions. Int Urol Nephrol. 2010; 42(1):141–150. PMID: 19582588.
Article13. Han WK, Bonventre JV. Biologic markers for the early detection of acute kidney injury. Curr Opin Crit Care. 2004; 10(6):476–482. PMID: 15616389.
Article14. Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003; 14(10):2534–2543. PMID: 14514731.
Article15. Ding H, He Y, Li K, Yang J, Li X, Lu R, Gao W. Urinary neutrophil gelatinase-associated lipocalin (NGAL) is an early biomarker for renal tubulointerstitial injury in IgA nephropathy. Clin Immunol. 2007; 123(2):227–234. PMID: 17360238.
Article16. Pedraza-Chaverrí J, González-Orozco AE, Maldonado PD, Barrera D, Medina-Campos ON, Hernández-Pando R. Diallyl disulfide ameliorates gentamicin-induced oxidative stress and nephropathy in rats. Eur J Pharmacol. 2003; 473(1):71–78. PMID: 12877940.
Article17. Chiarandini Fiore JP, Fanelli SL, de Ferreyra EC, Castro JA. Diallyl disulfide prevention of cis-Diamminedichloroplatinum-induced nephrotoxicity and leukopenia in rats: potential adjuvant effects. Nutr Cancer. 2008; 60(6):784–791. PMID: 19005978.18. Kim SH, Lee IC, Baek HS, Shin IS, Moon C, Bae CS, Kim SH, Kim JC, Kim HC. Mechanism for the protective effect of diallyl disulfide against cyclophosphamide acute urotoxicity in rats. Food Chem Toxicol. 2014; 64:110–118. PMID: 24291451.
Article19. Lee IC, Kim SH, Baek HS, Moon C, Kang SS, Kim SH, Kim YB, Shin IS, Kim JC. The involvement of Nrf2 in the protective effects of diallyl disulfide on carbon tetrachloride-induced hepatic oxidative damage and inflammatory response in rats. Food Chem Toxicol. 2014; 63:174–185. PMID: 24246655.
Article20. Das J, Ghosh J, Manna P, Sil PC. Taurine protects acetaminophen-induced oxidative damage in mice kidney through APAP urinary excretion and CYP2E1 inactivation. Toxicology. 2010; 269(1):24–34. PMID: 20067817.21. Kumanogoh A, Marukawa S, Suzuki K, Takegahara N, Watanabe C, Ch'ng E, Ishida I, Fujimura H, Sakoda S, Yoshida K, Kikutani H. Class IV semaphorin Sema4A enhances T-cell activation and interacts with Tim-2. Nature. 2002; 419(6907):629–633. PMID: 12374982.
Article22. Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, Freeman GJ, Kuchroo VK. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002; 415(6871):536–541. PMID: 11823861.
Article23. Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998; 273(7):4135–4142. PMID: 9461608.
Article24. Van Klinken BJ, Dekker J, Büller HA, Einerhand AW. Mucin gene structure and expression: protection vs. adhesion. Am J Physiol. 1995; 269:G613–G627. PMID: 7491952.
Article25. Xu S, Venge P. Lipocalins as biochemical markers of disease. Biochim Biophys Acta. 2000; 1482:298–307. PMID: 11058770.
Article26. Supavekin S, Zhang W, Kucherlapati R, Kaskel FJ, Moore LC, Devarajan P. Differential gene expression following early renal ischemia/reperfusion. Kidney Int. 2003; 63(5):1714–1724. PMID: 12675847.
Article27. Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol. 2006; 290(2):F517–F529. PMID: 16174863.
Article28. Hoffmann D, Adler M, Vaidya VS, Rached E, Mulrane L, Gallagher WM, Callanan JJ, Gautier JC, Matheis K, Staedtler F, Dieterle F, Brandenburg A, Sposny A, Hewitt P, Ellinger-Ziegelbauer H, Bonventre JV, Dekant W, Mally A. Performance of novel kidney biomarkers in preclinical toxicity studies. Toxicol Sci. 2010; 116(1):8–22. PMID: 20118187.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Exploring the role and mechanisms of diallyl trisulfide and diallyl disulfide in chronic constriction-induced neuropathic pain in rats
- Protective effect of diallyl disulfide on cyclophosphamide-induced testicular toxicity in rats
- A Case of Acetaminophen-induced Acute Interstitial Nephritis Presenting with Acute Renal Failure
- A Case of Acute Renal Failure Induced by Acetaminophen Intoxication
- The study of blood carbon disulfide in rats after oral administration of carbon disulfide