Immune Netw.
2016 Dec;16(6):366-372. 10.4110/in.2016.16.6.366.
Interleukin 17-expressing Innate Synovial Cells Drive K/Bxn Serum-induced Arthritis
- Affiliations
-
- 1Department of Anatomy & Cell Biology, College of Medicine, Hanyang University, Seoul 04763, Korea. jhyoun@hanyang.ac.kr
- 2Konkuk University Medical Center, Seoul 05030, Korea.
- KMID: 2362801
- DOI: http://doi.org/10.4110/in.2016.16.6.366
Abstract
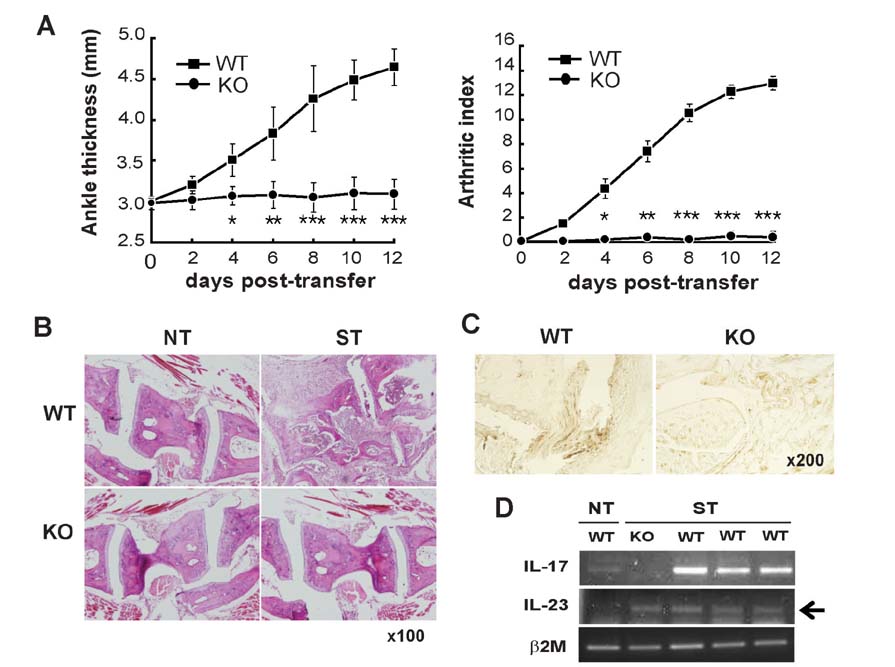
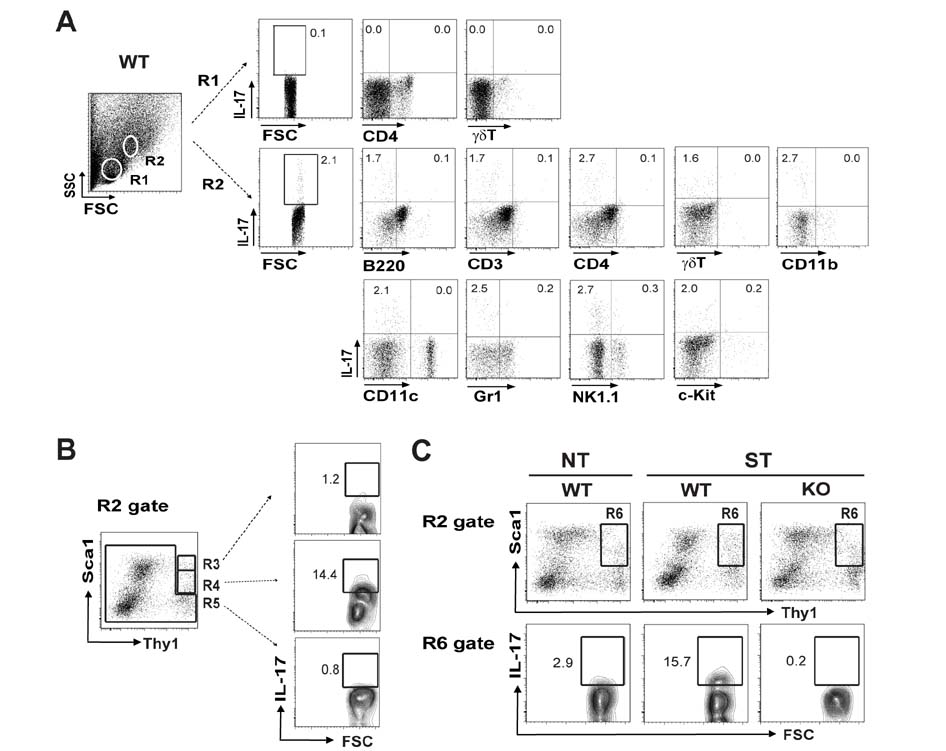
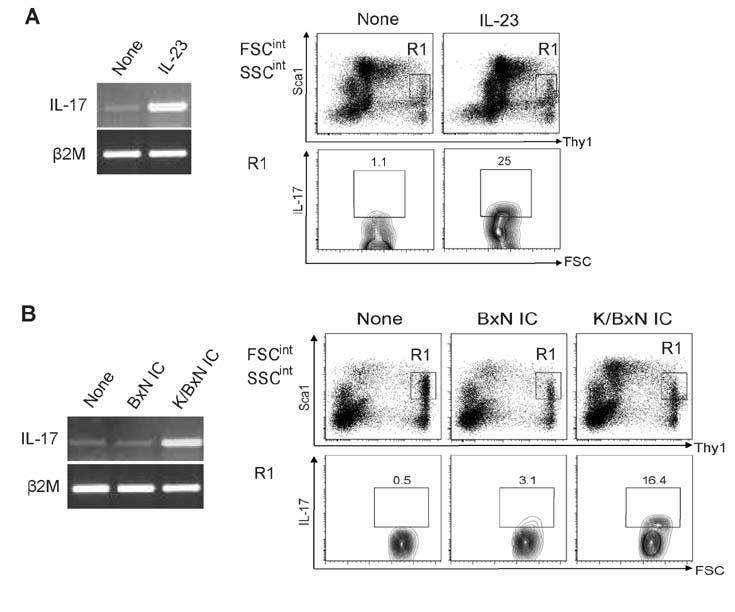
- K/BxN serum can induce arthritis in normal mice because of abundant autoantibodies that trigger an innate inflammatory response in joints. To determine whether IL-17 is involved in the pathogenesis of serum-induced arthritis, we injected wild-type and IL-17(−/−) mice with K/BxN serum and evaluated them for signs of arthritis. Unlike wild-type mice, IL-17(−/−) mice did not show any signs of arthritis. IL-17 was produced predominantly by CD3â» CD4â»Î³Î´TCRâ» NK1.1â» Sca1(int) Thy1(hi) cells residing in the inflamed synovial tissue. When synovial cells extracted from normal joints were stimulated with IL-23 or autoantibody-containing immune complexes, a substantial fraction of Sca1(int) Thy1(hi) cells produced IL-17. Thus, we have identified a novel population of IL-17-producing innate synovial cells that play a crucial role in the development of K/BxN serum-induced arthritis.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Aged Sanroque Mice Spontaneously Develop Sjögren's Syndrome-like Disease
Suk San Choi, Eunkyeong Jang, Yeon-Kyung Oh, Kiseok Jang, Mi-La Cho, Sung-Hwan Park, Jeehee Youn
Immune Netw. 2019;19(1):. doi: 10.4110/in.2019.19.e7.
Reference
-
1. Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001; 358:903–911.
Article2. Monach PA, Benoist C, Mathis D. The role of antibodies in mouse models of rheumatoid arthritis, and relevance to human disease. Adv Immunol. 2004; 82:217–248.
Article3. Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996; 87:811–822.
Article4. Korganow AS, Ji H, Mangialaio S, Duchatelle V, Pelanda R, Martin T, Degott C, Kikutani H, Rajewsky K, Pasquali JL, Benoist C, Mathis D. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999; 10:451–461.
Article5. Brennan FM, Maini RN, Feldmann M. Role of pro-inflammatory cytokines in rheumatoid arthritis. Springer Semin Immunopathol. 1998; 20:133–147.
Article6. Wipke BT, Allen PM. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J Immunol. 2001; 167:1601–1608.
Article7. Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010; 10:479–489.
Article8. Chabaud M, Durand JM, Buchs N, Fossiez F, Page G, Frappart L, Miossec P. Human interleukin-17: A T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999; 42:963–970.
Article9. Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N, Gillespie MT, Martin TJ, Suda T. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999; 103:1345–1352.
Article10. Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003; 171:6173–6177.
Article11. Pollinger B, Junt T, Metzler B, Walker UA, Tyndall A, Allard C, Bay S, Keller R, Raulf F, Di PF, O'Reilly T, Horwood NJ, Patel DD, Littlewood-Evans A. Th17 cells, not IL-17+ gammadelta T cells, drive arthritic bone destruction in mice and humans. J Immunol. 2011; 186:2602–2612.12. Ito Y, Usui T, Kobayashi S, Iguchi-Hashimoto M, Ito H, Yoshitomi H, Nakamura T, Shimizu M, Kawabata D, Yukawa N, Hashimoto M, Sakaguchi N, Sakaguchi S, Yoshifuji H, Nojima T, Ohmura K, Fujii T, Mimori T. Gamma/delta T cells are the predominant source of interleukin-17 in affected joints in collagen-induced arthritis, but not in rheumatoid arthritis. Arthritis Rheum. 2009; 60:2294–2303.
Article13. Jacobs JP, Wu HJ, Benoist C, Mathis D. IL-17-producing T cells can augment autoantibody-induced arthritis. Proc Natl Acad Sci U S A. 2009; 106:21789–21794.
Article14. Sadik CD, Kim ND, Alekseeva E, Luster AD. IL-17RA signaling amplifies antibody-induced arthritis. PLoS One. 2011; 6:e26342.
Article15. Jang E, Kim HR, Cho SH, Paik DJ, Kim JM, Lee SK, Youn J. Prevention of spontaneous arthritis by inhibiting homeostatic expansion of autoreactive CD4+ T cells in the K/BxN mouse mode. Arthritis Rheum. 2006; 54:492–498.
Article16. Jang E, Cho WS, Cho ML, Park HJ, Oh HJ, Kang SM, Paik DJ, Youn J. Foxp3+ regulatory T cells control humoral autoimmunity by suppressing the development of long-lived plasma cells. J Immunol. 2011; 186:1546–1553.
Article17. Lee H, Jin BE, Jang E, Lee AR, Han DS, Kim HY, Youn J. Gut-residing microbes alter the host susceptibility to autoantibody-mediated arthritis. Immune Netw. 2014; 14:38–44.
Article18. Oh YK, Jang E, Paik DJ, Youn J. Early growth response-1 plays a non-redundant role in the differentiation of B cells into plasma cells. Immune Netw. 2015; 15:161–166.
Article19. Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J Immunol. 2007; 178:4466–4472.
Article20. Lochner M, Peduto L, Cherrier M, Sawa S, Langa F, Varona R, Riethmacher D, Si-Tahar M, Di Santo JP, Eberl G. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma t+ T cells. J Exp Med. 2008; 205:1381–1393.
Article21. Rachitskaya AV, Hansen AM, Horai R, Li Z, Villasmil R, Luger D, Nussenblatt RB, Caspi RR. Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol. 2008; 180:5167–5171.
Article22. Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010; 464:1371–1375.
Article23. A versatile cell population. Nat Immunol. 2016; 17:754.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gut-residing Microbes Alter the Host Susceptibility to Autoantibody-mediated Arthritis
- Deficiency of Foxp3+ Regulatory T Cells Exacerbates Autoimmune Arthritis by Altering the Synovial Proportions of CD4+ T Cells and Dendritic Cells
- The Role of Type 2 Innate Lymphoid Cells in Allergic Diseases
- Lessons for the pathogenesis of rheumatoid arthritis acquired from experimental animal models
- Induction of Apoptosis in Synovial Cells from Patients with Rheumatoid Arthritis