Cancer Res Treat.
2016 Oct;48(4):1253-1263. 10.4143/crt.2015.400.
Prognostic Scoring Index for Patients with Metastatic Pancreatic Adenocarcinoma
- Affiliations
-
- 1Department of Pharmacology and Brain Korea 21 Plus Project for Medical Sciences, Yonsei University College of Medicine, Seoul, Korea.
- 2Biostatistics Collaboration Unit, Yonsei University College of Medicine, Seoul, Korea.
- 3Cancer Prevention Center, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea.
- 4Pancreatobiliary Cancer Clinic, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. jeunghc1123@yuhs.ac
- 5Songdang Institute for Cancer Research, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2356227
- DOI: http://doi.org/10.4143/crt.2015.400
Abstract
- PURPOSE
This study focused on implementation of a prognostic scoring index based on clinico-laboratory parameters measured routinely on admission in metastatic pancreatic cancer patients.
MATERIALS AND METHODS
Records from 403 patients of metastatic disease were analyzed retrospectively. Continuous variables were dichotomized according to the normal range or the best cut-off values statistically determined by Contal and O'Quigley method, and then analyzed in association with prognosis"”overall survival (OS), using Cox's proportional hazard model. Scores were calculated by summing the rounded chi-square scores for the factors that emerged in the multivariate analysis.
RESULTS
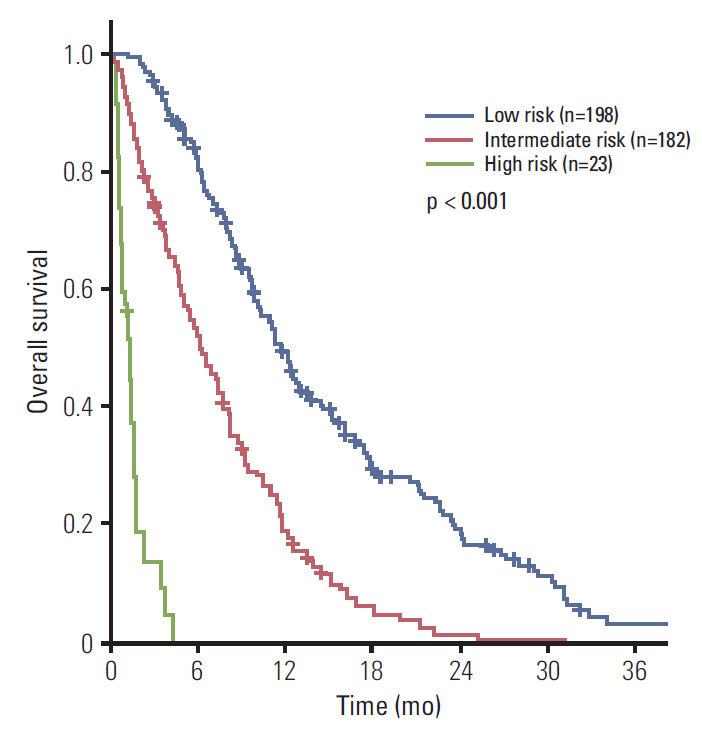
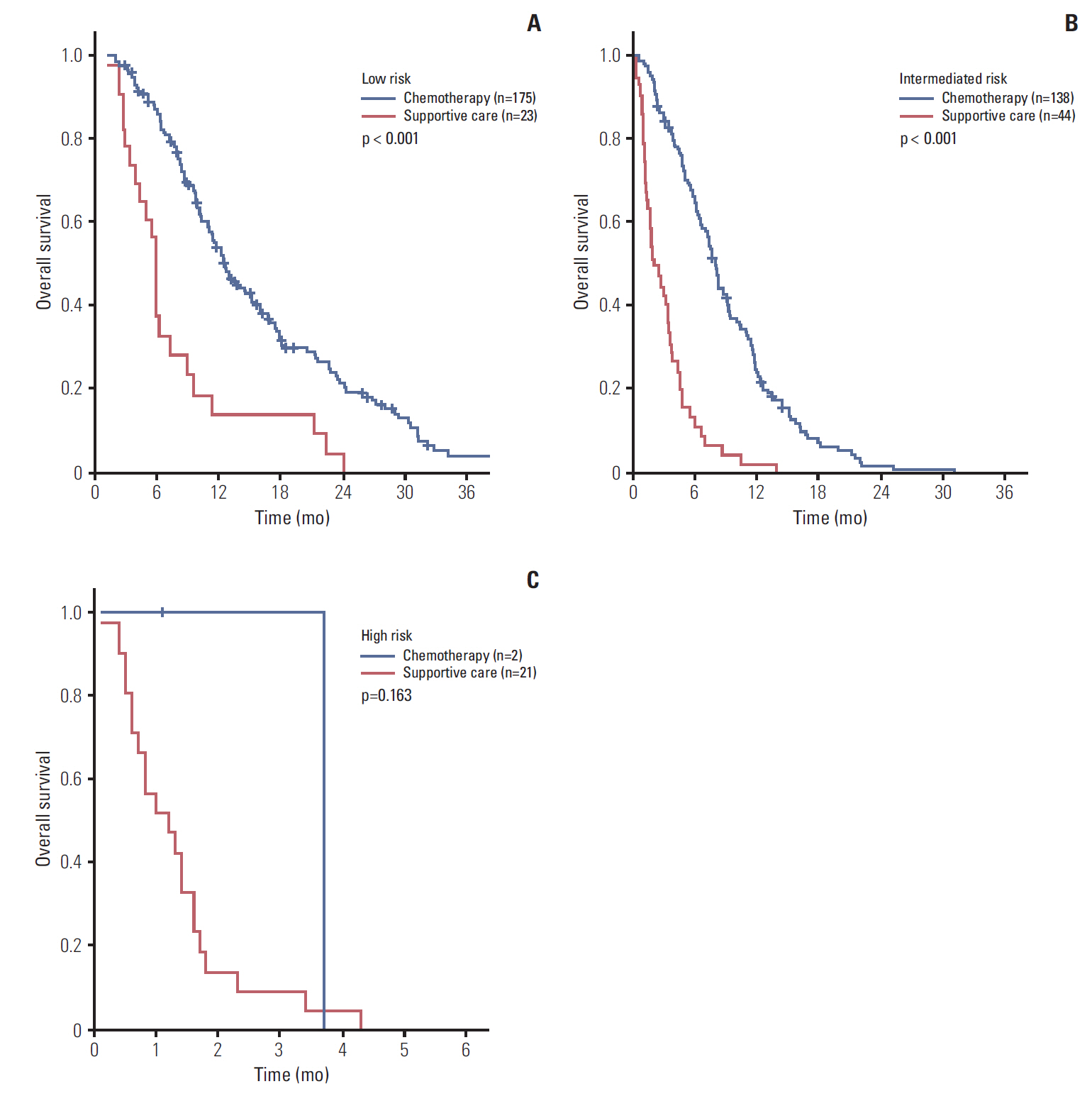
Performance status, hemoglobin, leucocyte count, neutrophil-lymphocyte ratio, and carcinoembryonic antigen were independent factors for OS. When patients were divided into three risk groups according to these factors, median survival was 11.7, 6.2, and 1.3 months for the low, intermediate, and high-risk groups, respectively (p < 0.001). Palliative chemotherapy has a significant survival benefit for low and intermediate-risk patients (median OS; 12.5 months vs. 5.9 months, p < 0.001 and 8.0 months vs. 2.0 months, p < 0.001, respectively).
CONCLUSION
We advocate the use of a multivariable approach with continuous variables for prognostic modeling. Our index is helpful in accurate patient risk stratification and may aid in treatment selection.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Declined Preoperative Aspartate Aminotransferase to Neutrophil Ratio Index Predicts Poor Prognosis in Patients with Intrahepatic Cholangiocarcinoma after Hepatectomy
Lingyun Liu, Wei Wang, Yi Zhang, Jianting Long, Zhaohui Zhang, Qiao Li, Bin Chen, Shaoqiang Li, Yunpeng Hua, Shunli Shen, Baogang Peng
Cancer Res Treat. 2018;50(2):538-550. doi: 10.4143/crt.2017.106.
Reference
-
References
1. Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014; 371:2140–1.
Article2. Ni XG, Bai XF, Mao YL, Shao YF, Wu JX, Shan Y, et al. The clinical value of serum CEA, CA19-9, and CA242 in the diagnosis and prognosis of pancreatic cancer. Eur J Surg Oncol. 2005; 31:164–9.
Article3. Stocken DD, Hassan AB, Altman DG, Billingham LJ, Bramhall SR, Johnson PJ, et al. Modelling prognostic factors in advanced pancreatic cancer. Br J Cancer. 2008; 99:883–93.
Article4. Yi JH, Lee J, Park SH, Lee KT, Lee JK, Lee KH, et al. A prognostic model to predict clinical outcomes with first-line gemcitabine-based chemotherapy in advanced pancreatic cancer. Oncology. 2011; 80:175–80.
Article5. Ciela B. Hematology in practice. 2nd ed. Philadelphia, PA: F.A. Davis Company;2011.6. Contal C, O'Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput Stat Data Anal. 1999; 30:253–70.
Article7. Eo SH, Hong SM, Cho HY. K-adaptive partitioning for survival data: the Kaps add-on package for R. arXiv:1306.4615. Ithaca, NY: arXiv, Cornell University Library;2013.8. Harrell FE Jr, Lee KL, Matchar DB, Reichert TA. Regression models for prognostic prediction: advantages, problems, and suggested solutions. Cancer Treat Rep. 1985; 69:1071–7.9. Ziske C, Schlie C, Gorschluter M, Glasmacher A, Mey U, Strehl J, et al. Prognostic value of CA 19-9 levels in patients with inoperable adenocarcinoma of the pancreas treated with gemcitabine. Br J Cancer. 2003; 89:1413–7.
Article10. Lee KJ, Yi SW, Chung MJ, Park SW, Song SY, Chung JB, et al. Serum CA 19-9 and CEA levels as a prognostic factor in pancreatic adenocarcinoma. Yonsei Med J. 2013; 54:643–9.
Article11. Ballehaninna UK, Chamberlain RS. Serum CA 19-9 as a biomarker for pancreatic cancer: a comprehensive review. Indian J Surg Oncol. 2011; 2:88–100.12. Mazumdar M, Glassman JR. Categorizing a prognostic variable: review of methods, code for easy implementation and applications to decision-making about cancer treatments. Stat Med. 2000; 19:113–32.
Article13. Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: an evidence based appraisal. J Gastrointest Oncol. 2012; 3:105–19.14. Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2009; 137:425–8.
Article15. Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005; 91:181–4.
Article16. Fock RA, Blatt SL, Beutler B, Pereira J, Tsujita M, de Barros FE, et al. Study of lymphocyte subpopulations in bone marrow in a model of protein-energy malnutrition. Nutrition. 2010; 26:1021–8.
Article17. Palucka K, Coussens LM, O'Shaughnessy J. Dendritic cells, inflammation, and breast cancer. Cancer J. 2013; 19:511–6.
Article18. Luo G, Guo M, Liu Z, Xiao Z, Jin K, Long J, et al. Blood neutrophil-lymphocyte ratio predicts survival in patients with advanced pancreatic cancer treated with chemotherapy. Ann Surg Oncol. 2015; 22:670–6.
Article19. Garcea G, Ladwa N, Neal CP, Metcalfe MS, Dennison AR, Berry DP. Preoperative neutrophil-to-lymphocyte ratio (NLR) is associated with reduced disease-free survival following curative resection of pancreatic adenocarcinoma. World J Surg. 2011; 35:868–72.
Article20. Sugiura T, Uesaka K, Kanemoto H, Mizuno T, Okamura Y. Elevated preoperative neutrophil-to-lymphocyte ratio as a predictor of survival after gastroenterostomy in patients with advanced pancreatic adenocarcinoma. Ann Surg Oncol. 2013; 20:4330–7.
Article21. Hostetter RB, Augustus LB, Mankarious R, Chi KF, Fan D, Toth C, et al. Carcinoembryonic antigen as a selective enhancer of colorectal cancer metastasis. J Natl Cancer Inst. 1990; 82:380–5.
Article22. Yasue M, Sakamoto J, Teramukai S, Morimoto T, Yasui K, Kuno N, et al. Prognostic values of preoperative and postoperative CEA and CA19.9 levels in pancreatic cancer. Pancreas. 1994; 9:735–40.
Article23. Ruiz-Tovar J, Martin-Perez E, Fernandez-Contreras ME, Reguero-Callejas ME, Gamallo-Amat C. Identification of prognostic factors in pancreatic cancer. Cir Cir. 2011; 79:313–22.24. Ghaneh P, Kawesha A, Evans JD, Neoptolemos JP. Molecular prognostic markers in pancreatic cancer. J Hepatobiliary Pancreat Surg. 2002; 9:1–11.
Article25. Keam B, Kim DW, Park JH, Lee JO, Kim TM, Lee SH, et al. Nomogram predicting clinical outcomes in non-small cell lung cancer patients treated with epidermal growth factor receptor tyrosine kinase inhibitors. Cancer Res Treat. 2014; 46:323–30.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prognostic value of COL6A3 in pancreatic adenocarcinoma
- Clinical importance of preoperative and postoperative prognostic nutritional index in patients with pancreatic ductal adenocarcinoma
- Metastatic Gastric Adenocarcinoma of The Lower Eyelid
- A Case of Skin Metastasis Manifested as a Presenting Sign of Pancreatic Tail Cancer
- Two Cases of Supraclavicular Lymph Node Metastasis in Pancreatic Adenocarcinoma