J Cerebrovasc Endovasc Neurosurg.
2016 Sep;18(3):258-263. 10.7461/jcen.2016.18.3.258.
Emergent Double-barrel Bypass Shortly after Intravenous Administration of Recombinant Tissue Plasminogen Activator for Acute Ischemic Stroke
- Affiliations
-
- 1Department of Neurosurgery, Busan-Ulsan Regional Cardio-Cerebrovascular Center, Medical Science Research Center, College of Medicine, Dong-A University, Busan, Korea. nsparkhs@dau.ac.kr
- KMID: 2355651
- DOI: http://doi.org/10.7461/jcen.2016.18.3.258
Abstract
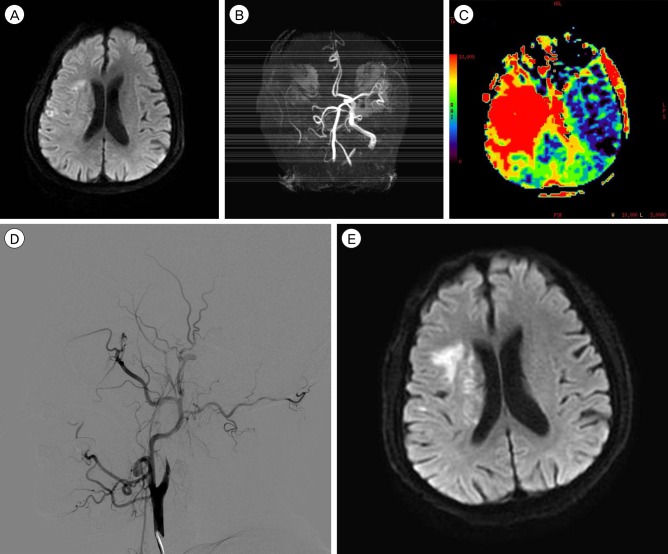
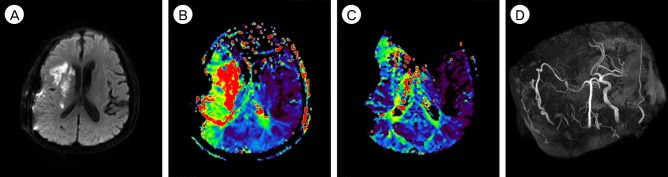
- Although intravenous recombinant tissue plasminogen activator (IV rt-PA) is effective in many cases of acute ischemic stroke, the neurologic symptoms can worsen after IV rt-PA because of sustained vessel occlusion. For such cases, several reperfusion modalities are available, including intra-arterial thrombolysis (IAT), carotid endarterectomy, and superficial temporal artery-middle cerebral artery (STA-MCA) bypass. Invasive procedures, such as major surgery, should be generally avoided within 24 hours after the administration of IV rt-PA. A 66-year-old man with no previous medical history developed left hemiparesis. A computed tomography scan revealed no acute lesion and he received IV rt-PA within 1.5 hours after symptom onset. Emergent magnetic resonance imaging showed significant diffusion-perfusion mismatch. He received IAT 2 hours after IV rt-PA administration, but IAT failed because of total occlusion of the cervical internal carotid artery. We initially planned to perform STA-MCA bypass the next morning because he had received IV rt-PA, but, 8 hours after IV rt-PA administration, his hemiparesis worsened from motor grade 3/4 to motor grade 1/2. Because of the large perfusion defect in both MCA divisions, double-barrel STA-MCA bypass was performed 10 hours after IV rt-PA administration. His symptoms rapidly improved after surgery and his modified Rankin Scale score 3 months later was grade 0. We suggest that emergent double-barrel bypass can be a viable option in patients who have perfusion defects of both MCA divisions in acute ischemic stroke after IV rt-PA administration.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Revision Superficial Temporal Artery-Middle Cerebral Artery Bypass Surgery for Recurrent Acute Ischemic Stroke Due to Delayed Occlusion of the Bypass Graft
Yun-Hee Choi, Hyun-Seok Park, Myong-Jin Kang, Jae-Kwan Cha
J Cerebrovasc Endovasc Neurosurg. 2018;20(2):127-132. doi: 10.7461/jcen.2018.20.2.127.
Reference
-
1. Adams HP Jr, Bendixen BH, Leira E, Chang KC, Davis PH, Woolson RF, et al. Antithrombotic treatment of ischemic stroke among patients with occlusion or severe stenosis of the internal carotid artery: A report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology. 1999; 7. 53(1):122–125. PMID: 10408547.
Article2. Bhatia R, Hill MD, Shobha N, Menon B, Bal S, Kochar P, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke. 2010; 10. 41(10):2254–2258. PMID: 20829513.3. Choi JY, Lee JI, Lee TH, Sung SM, Cho HJ, Ko JK. Emergent recanalization with stenting for acute stroke due to athero-thrombotic occlusion of the cervical internal carotid artery : a single center experience. J Korean Neurosurg Soc. 2014; 6. 55(6):313–320. PMID: 25237426.
Article4. Duckworth EA, Rao VY, Patel AJ. Double-barrel bypass for cerebral ischemia: technique, rationale, and preliminary experience with 10 consecutive cases. Neurosurgery. 2013; 9. 73(1 Suppl Operative):ons30–ons38. discussion ones37-8. PMID: 23313980.
Article5. Garrett MC, Komotar RJ, Starke RM, Merkow MB, Otten ML, Sciacca RR, et al. The efficacy of direct extracranial-intracranial bypass in the treatment of symptomatic hemodynamic failure secondary to athero-occlusive disease: a systematic review. Clin Neurol Neurosurg. 2009; 5. 111(4):319–326. PMID: 19201526.
Article6. Hayashi T, Shirane R, Fujimura M, Tominaga T. Postoperative neurological deterioration in pediatric moyamoya disease: watershed shift and hyperperfusion. J Neurosurg Pediatr. 2010; 6(1):73–81. PMID: 20593991.
Article7. Horiuchi T, Nitta J, Ishizaka S, Kanaya K, Yanagawa T, Hongo K. Emergency EC-IC bypass for symptomatic atherosclerotic ischemic stroke. Neurosurg Rev. 2013; 10. 36(4):559–564. discussion 564-5. PMID: 23821132.
Article8. Hwang G, Oh CW, Bang JS, Jung CK, Kwon OK, Kim JE, et al. Superficial temporal artery to middle cerebral artery bypass in acute ischemic stroke and stroke in progress. Neurosurgery. 2011; 3. 68(3):723–729. discussion 729-30. PMID: 21311299.
Article9. Ishishita Y, Kimura T, Morita A. Urgent superficial temporal artery to middle cerebral artery bypass shortly after intravenous rt-PA. Br J Neurosurg. 2012; 10. 26(5):773–775. PMID: 22463811.
Article10. Lee SB, Huh PW, Kim DS, Yoo DS, Lee TG, Cho KS. Early superficial temporal artery to middle cerebral artery bypass in acute ischemic stroke. Clin Neurol Neurosurg. 2013; 8. 115(8):1238–1244. PMID: 23266265.
Article11. McPherson CM, Woo D, Cohen PL, Pancioli AM, Kissela BM, Carrozzella JA, et al. Early carotid endarterectomy for critical carotid artery stenosis after thrombolysis therapy in acute ischemic stroke in the middle cerebral artery. Stroke. 2001; 9. 32(9):2075–2080. PMID: 11546899.
Article12. Molina CA, Chamorro A, Rovira A, de Miquel A, Serena J, Roman LS, et al. REVASCAT: a randomized trial of revascularization with SOLITAIRE FR device vs. best medical therapy in the treatment of acute stroke due to anterior circulation large vessel occlusion presenting within eight-hours of symptom onset. Int J Stroke. 2015; 6. 10(4):619–626. PMID: 24206399.
Article13. Nussbaum ES, Janjua TM, Defillo A, Lowary JL, Nussbaum LA. Emergency extracranial-intracranial bypass surgery for acute ischemic stroke. J Neurosurg. 2010; 3. 112(3):666–673. PMID: 19499983.
Article14. Powers WJ, Clarke WR, Grubb RL Jr, Videen TO, Adams HP Jr, Derdeyn CP, et al. Extracranial-intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia: the Carotid Occlusion Surgery Study randomized trial. JAMA. 2011; 306(18):1983–1992. PMID: 22068990.15. Sakai K, Nitta J, Horiuchi T, Ogiwara T, Kobayashi S, Tanaka Y, et al. Emergency revascularization for acute main-trunk occlusion in the anterior circulation. Neurosurg Rev. 2008; 1. 31(1):69–76. discussion 76. PMID: 17957395.
Article16. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015; 6. 372(24):2285–2295. PMID: 25882376.
Article17. Takeuchi S, Wada K, Arimoto H, Kumagai K, Osada H, Otani N, et al. Emergency superficial temporal artery to middle cerebral artery bypass after intravenous administration of tissue plasminogen activator for stroke. Turk Neurosurg. 2015; 25(4):633–637. PMID: 26242342.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment of Acute Ischemic Stroke: Thrombolysis
- Intravenous Thrombolysis and Endovascular Thrombectomy in Acute Ischemic Stroke with Minor Symptom
- Unexpected Complication of Intravenous Recombinant Tissue Plasminogen Activator Thrombolysis in a Patient with Acute Ischemic Stroke: Aortic Dissection
- ST-Elevation Myocardial Infarction after Intravenous Thrombolysis Treatment for Acute Ischemic Stroke
- Recent advances in thrombolysis of acute ischemic stroke