J Korean Med Sci.
2016 Dec;31(12):1969-1975. 10.3346/jkms.2016.31.12.1969.
Development and Validation of the Korean Version of Hand-Foot Skin Reaction and Quality of Life Questionnaire (HF-QoL-K)
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea. taejong.song@gmail.com
- 2Department of Obstetrics and Gynecology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Obstetrics and Gynecology, Chonnam National University Hwasun Hospital, Chonnam National University Medical School, Hwasun, Korea.
- 4Gynecologic Cancer Branch and Center for Uterine Cancer, Research Institute and Hospital, National Cancer Center, Goyang, Korea.
- 5Korean Language and Literature, Kyung Hee University, Seoul, Korea.
- KMID: 2355627
- DOI: http://doi.org/10.3346/jkms.2016.31.12.1969
Abstract
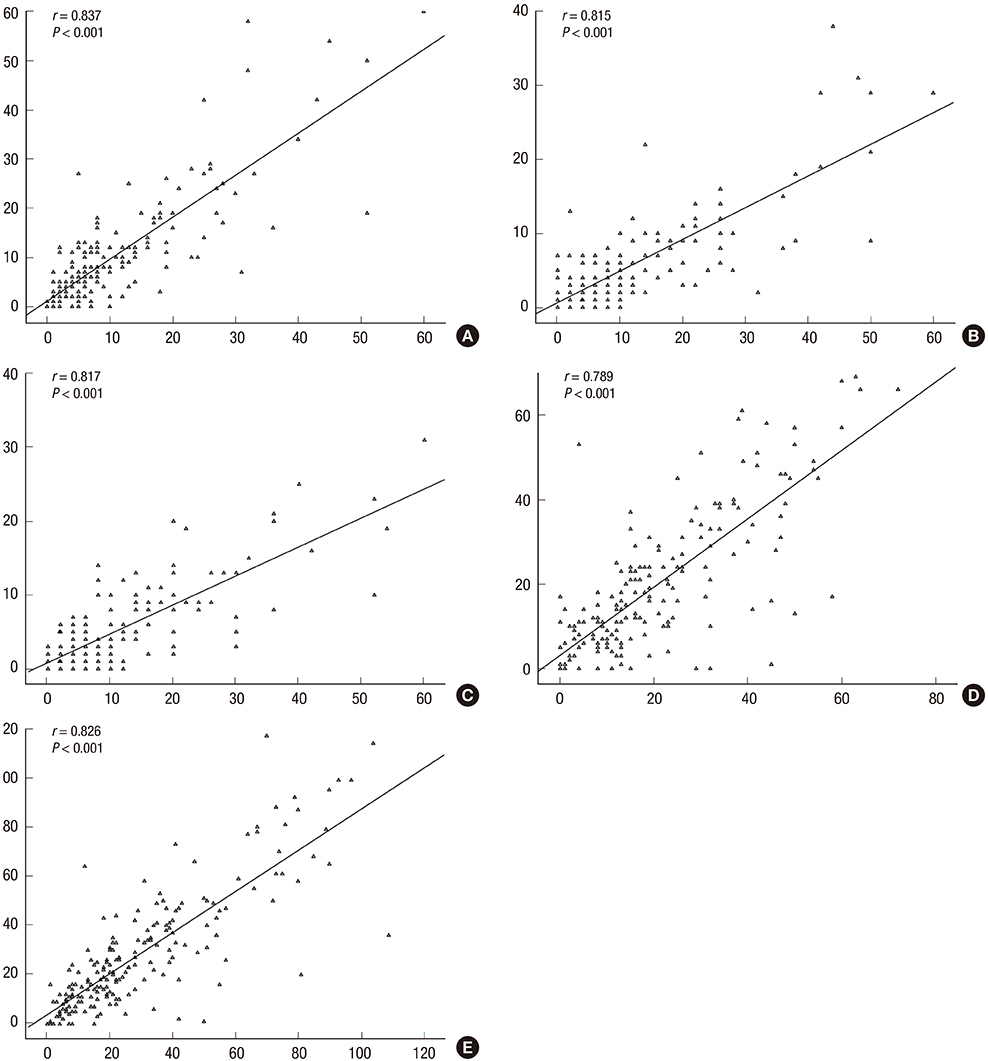
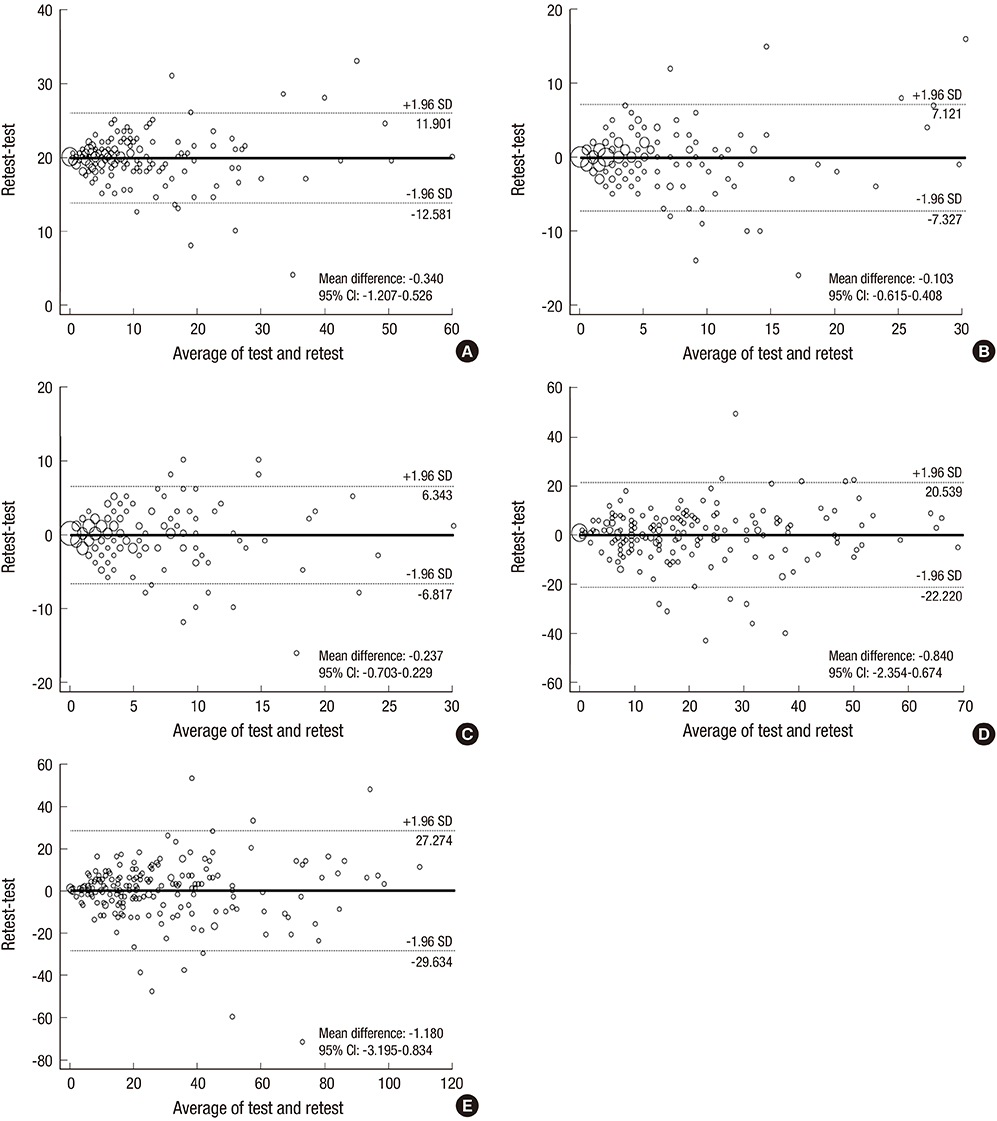
- Given the growing number of cancer patients and the resulting increase in the administration of chemotherapeutic agents, convenient and effective methods for measuring the symptoms and quality of life associated with the hand-foot syndrome (HFS) are needed. Therefore, the aim of this study was to develop and validate the Korean version of the hand-foot skin reaction and quality of life questionnaire (HF-QoL-K), comprising a 20-item symptom domain and an 18-item daily activity domain. After we developed the HF-QoL-K, 209 Korean patients with gynecologic cancer who were undergoing chemotherapeutic agents relating the HFS were asked to fill in the questionnaire. The content validity, internal consistency reliability, and test-retest reliability were evaluated. The internal validity index, Cronbach's alpha coefficient, and intra-class correlation coefficient of the HF-QoL-K were 0.90, 0.958, and 0.825 (95% confidence interval [CI], 0.774-0.865), respectively. The scatter plot (Pearson correlation coefficient, 0.826) and the Bland-Altman plot for test-retest reliability were also acceptable. The HF-QoL-K instrument is a valid and reliable questionnaire for the measurement of the symptoms and quality of life in Korean cancer patients suffering HFS.
Keyword
Figure
Cited by 1 articles
-
Therapeutic Effect of Anti-inflammatory Tripeptide Cream in Hand-Foot Syndrome/Skin Reaction Related to Anticancer Drugs: A Randomized, Double-Blind, Placebo-Controlled Pilot Trial
Yaewon Yang, Jang-Hee Hahn, Min Seo Kim, Minkwan Jo, Yong-Pyo Lee, Hongsik Kim, Hee Kyung Kim, Jihyun Kwon, Ki Hyeong Lee, Hye Sook Han
Cancer Res Treat. 2024;56(4):1050-1057. doi: 10.4143/crt.2024.080.
Reference
-
1. Clark AS, Vahdat LT. Chemotherapy-induced palmar-plantar erythrodysesthesia syndrome: etiology and emerging therapies. Support Cancer Ther. 2004; 1:213–218.2. Chen M, Zhang L, Wang Q, Shen J. Pyridoxine for prevention of hand-foot syndrome caused by chemotherapy: a systematic review. PLoS One. 2013; 8:e72245.3. Autier J, Escudier B, Wechsler J, Spatz A, Robert C. Prospective study of the cutaneous adverse effects of sorafenib, a novel multikinase inhibitor. Arch Dermatol. 2008; 144:886–892.4. Macedo LT, Lima JP, dos Santos LV, Sasse AD. Prevention strategies for chemotherapy-induced hand-foot syndrome: a systematic review and meta-analysis of prospective randomised trials. Support Care Cancer. 2014; 22:1585–1593.5. Lacouture ME, Wu S, Robert C, Atkins MB, Kong HH, Guitart J, Garbe C, Hauschild A, Puzanov I, Alexandrescu DT, et al. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist. 2008; 13:1001–1011.6. Anderson RT, Keating KN, Doll HA, Camacho F. The hand-foot skin reaction and quality of life questionnaire: an assessment tool for oncology. Oncologist. 2015; 20:831–838.7. Floyd FJ, Widaman KF. Factor analysis in the development and refinement of clinical assessment instruments. Psychol Assess. 1995; 7:286–299.8. Oh CM, Won YJ, Jung KW, Kong HJ, Cho H, Lee JK, Lee DH, Lee KH. Community of Population-Based Regional Cancer Registries. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2013. Cancer Res Treat. 2016; 48:436–450.9. Son M, Yun JW. Cancer mortality projections in Korea up to 2032. J Korean Med Sci. 2016; 31:892–901.10. Degen A, Alter M, Schenck F, Satzger I, Völker B, Kapp A, Gutzmer R. The hand-foot-syndrome associated with medical tumor therapy - classification and management. J Dtsch Dermatol Ges. 2010; 8:652–661.11. Nagore E, Insa A, Sanmartín O. Antineoplastic therapy-induced palmar plantar erythrodysesthesia (‘hand-foot’) syndrome. Incidence, recognition and management. Am J Clin Dermatol. 2000; 1:225–234.12. Webster-Gandy JD, How C, Harrold K. Palmar-plantar erythrodysesthesia (PPE): a literature review with commentary on experience in a cancer centre. Eur J Oncol Nurs. 2007; 11:238–246.13. Farr KP, Safwat A. Palmar-plantar erythrodysesthesia associated with chemotherapy and its treatment. Case Rep Oncol. 2011; 4:229–235.14. U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute, Division of Cancer Treatment and Diagnosis. Cancer therapy evaluation program (CTEP): common terminology criteria for adverse events, version 3.0. March 31, 2003. accessed on 9 August 2006. Available at http://ctep.cancer.gov.15. Sibaud V, Dalenc F, Chevreau C, Roché H, Delord JP, Mourey L, Lacaze JL, Rahhali N, Taïeb C. HFS-14, a specific quality of life scale developed for patients suffering from hand-foot syndrome. Oncologist. 2011; 16:1469–1478.16. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982; 5:649–655.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Computerized Measurement for Asthma-Specific Quality of Life: Comparison with a Conventional Paper-and-Pencil Questionnaire
- Translation and Linguistic Validation of the Korean Version of the Wisconsin Stone Quality of Life Questionnaire
- Therapeutic Effect of Anti-inflammatory Tripeptide Cream in Hand-Foot Syndrome/Skin Reaction Related to Anticancer Drugs: A Randomized, Double-Blind, Placebo-Controlled Pilot Trial
- Skin-Related Toxicity of Tyrosine Kinase Inhibitor in Thyroid Cancer
- Severity of Hand Dermatitis and Quality of Life in Nurses