Perinatology.
2016 Sep;27(3):162-167. 10.14734/PN.2016.27.3.162.
Early Prophylactic Use of Curosurf® Versus Newfactan® for Respiratory Distress Syndrome in Premature Infants
- Affiliations
-
- 1Department of Pediatrics, Ajou University School of Medicine, Suwon, Korea. neopedlee@gmail.com
- KMID: 2355574
- DOI: http://doi.org/10.14734/PN.2016.27.3.162
Abstract
- PURPOSE
This study was done to compare the prophylactic efficacy of surfactant with Curosurf® and Newfactan®.
METHODS
Preterm infants treated with pulmonary surfactant within 30 minutes after birth from January 2008 to December 2014 were included in this study. The subjects were divided into a group that received Curosurf® (n=163) and a group that received Newfactan® (n=120). The data were collected retrospectively using the patients' medical records.
RESULTS
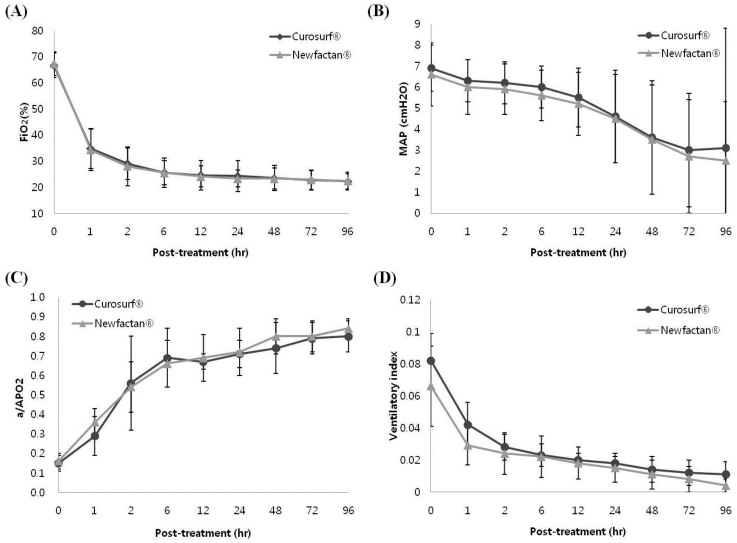
Demographic factors did not differ between the groups. The incidence rates of surfactant re-dosing, pulmonary air leaks, postnatal steroid therapy use, pulmonary hemorrhage, moderate to severe bronchopulmonary dysplasia, and duration of mechanical ventilation, were not different between the groups. The sequential changes of ventilator parameters after surfactant instillation were not different between the groups. No differences in the incidence rates of patent ductus arteriosus, intraventricular hemorrhage (≥grade III), periventricular leukomalacia, retinopathy of prematurity, necrotizing enterocolitis (≥stage IIa), mortality, sepsis, or duration of hospital stay were observed between the groups.
CONCLUSION
The results indicate no differences in the clinical efficacy of early prophylactic use of Curosurf® versus Newfactan®.
MeSH Terms
-
Bronchopulmonary Dysplasia
Demography
Ductus Arteriosus, Patent
Enterocolitis, Necrotizing
Hemorrhage
Humans
Incidence
Infant, Newborn
Infant, Premature*
Length of Stay
Leukomalacia, Periventricular
Medical Records
Mortality
Parturition
Pulmonary Surfactants
Respiration, Artificial
Retinopathy of Prematurity
Retrospective Studies
Sepsis
Treatment Outcome
Ventilators, Mechanical
Pulmonary Surfactants
Figure
Reference
-
1. Jobe AH. Pulmonary surfactant therapy. N Engl J Med. 1993; 328:861–868.
Article2. Sandberg KL, Lindstrom DP, Sjöqvist BA, Parker RA, Cotton RB. Surfactant replacement therapy improves ventilation inhomogeneity in infants with respiratory distress syndrome. Pediatr Pulmonol. 1997; 24:337–343.
Article3. Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants-2010 update. Zhonghua Er Ke Za Zhi. 2011; 49:27–33.4. Engle WA. Surfactant-replacement therapy for respiratory distress in the preterm and term neonate. Pediatrics. 2008; 121:419–432.
Article5. Polin RA, Carlo WA. Surfactant replacement therapy for preterm and term neonates with respiratory distress. Pediatrics. 2014; 133:156–163.
Article6. Halliday HL. Surfactants: past, present and future. J Perinatol. 2008; 28:Suppl 1. S47–S56.
Article7. Pramanik AK, Holtzman RB, Merritt TA. Surfactant replacement therapy for pulmonary diseases. Pediatr Clin North Am. 1993; 40:913–936.
Article8. Soll RF, Morley CJ. Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2001; (2):CD000510.
Article9. Fujiwara T, Maeta H, Chida S, Morita T, Watabe Y, Abe T. Artificial surfactant therapy in hyaline-membrane disease. Lancet. 1980; 1:55–59.
Article10. Choi CW, Hwang JH, Yoo EJ, Kim KA, Koh SY, Lee YK, et al. Comparison of clinical efficacy of Newfactan® versus Surfacten® for the treatment of respiratory distress syndrome in the newborn infants. J Korean Med Sci. 2005; 20:591–597.
Article11. Jeon GW, Oh M, Sin JB. Efficacy of surfactant-TA, calfactant and poractant alfa for preterm infants with respiratory distress syndrome: a retrospective study. Yonsei Med J. 2015; 56:433–439.
Article12. Kim SM, Park YJ, Chung SH, Choi YS, Kim CH, Bae CW. Early prophylactic versus late selective use of surfactant for respiratory distress syndrome in very preterm infants: a collaborative study of 53 multi-center trials in Korea. J Korean Med Sci. 2014; 29:1126–1131.
Article13. Kim JN, Shim EJ. Comparison of Curosurf® versus Surfacten® in the treatment of respiratory distress syndrome. Neonatal Med. 2013; 20:207–213.
Article14. Bae CW, Hahn WH, Chang JY, Kim SM. Surfactant replacement therapy for RDS: a collaborative study of 72 multi-center trials in Korea (2010) and a review of Korean experiences over 20 years. J Korean Soc Neonatol. 2011; 18:409–411.
Article15. Chung KY, Lee NM, Yun SW, Chae SA, Lim IS, Choi ES, et al. Comparison of outcomes between prophylactic and rescue therapy of surfactant in premature infants. Neonatal Med. 2013; 20:90–96.
Article16. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001; 163:1723–1729.
Article17. Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986; 33:179–201.
Article18. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978; 92:529–534.
Article19. Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988; 16:128–140.
Article20. Choi BM, Lee KH, Eun BL, Yoo KH, Hong YS, Son CS, et al. Utility of rapid B-type natriuretic peptide assay for diagnosis of symptomatic patent ductus arteriosus in preterm infants. Pediatrics. 2005; 115:e255–e261.
Article21. Egberts J, de Winter JP, Sedin G, de Kleine MJ, Broberger U, van Bel F, et al. Comparison of prophylaxis and rescue treatment with Curosurf® in neonates less than 30 weeks' gestation: a randomized trial. Pediatrics. 1993; 92:768–774.
Article22. Ramanathan R. Animal-derived surfactants: where are we? the evidence from randomized, controlled clinical trials. J Perinatol. 2009; 29:Suppl 2. S38–S43.
Article23. Fujii AM, Patel SM, Allen R, Doros G, Guo CY, Testa S. Poractant alfa and beractant treatment of very premature infants with respiratory distress syndrome. J Perinatol. 2010; 30:665–670.
Article24. Trembath A, Hornik CP, Clark R, Smith PB, Daniels J, Laughon M. Comparative effectiveness of surfactant preparations in premature infants. J Pediatr. 2013; 163:955–960.
Article25. Hong SW, Lee EH, Kim SY, Park HJ. Comparison of the therapeutic effects of Curosurf® and Newfactan® in respiratory distress syndrome. J Korean Soc Neonatol. 2008; 15:142–150.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Respiratory Distress Syndrome on the Thyroid Function
- Early Prophylactic versus Late Selective Use of Surfactant for Respiratory Distress Syndrome in Very Preterm Infants: A Collaborative Study of 53 Multi-Center Trials in Korea
- The Importance and the Need of Early Pulmonary Surfactant Therapy in Premature Infant with Respiratory Distress Syndrome
- Surfactant Therapy in Respiratory Distress Syndrome
- The Effects of Open Endotracheal Suctioning(ETS) and Close ETS on Oxygen Saturation and Heart Rate in Premature Infants with Respiratory Distress Syndrome