Clin Nutr Res.
2016 Oct;5(4):219-236. 10.7762/cnr.2016.5.4.219.
The Efficacy of Oral Nutritional Intervention in Malnourished Cancer Patients: a Systemic Review
- Affiliations
-
- 1Department of Food and Nutrition, Sookmyung Women's University, Seoul 04310, Korea. mksung@sm.ac.kr
- KMID: 2355295
- DOI: http://doi.org/10.7762/cnr.2016.5.4.219
Abstract
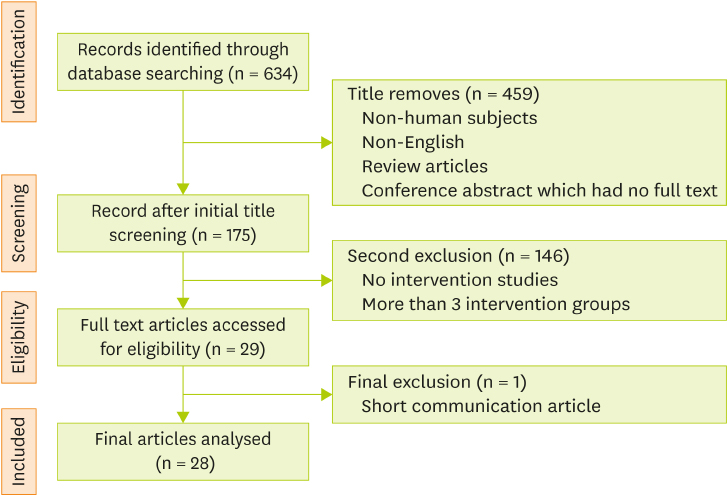
- Cancer is currently a leading cause of deaths worldwide and the number of new cases is growing rapidly in both, developed and developing countries. Nutritional management during and after cancer treatment affects treatment efficacy and patient quality of life (QOL). This review systemically examined the effect of oral nutritional interventions on nutritional and clinical outcomes in cancer patients. We especially focused on outcomes such as nutritional status indices, immune-associated biochemical markers, and QOL assessments to provide insights on the applicability of different outcomes. A total of 28 papers were selected for systematic review. The nutritional composition of oral nutritional supplements (ONS), outcome measures, and efficacy of the oral nutritional interventions were summarized and discussed. Most ONS contain 1 or more functional components in addition to basic nutrients. Each study used various outcome measures and significant efficacy was observed for a limited number of measures. Nutritional status indices, QOL measures, and the duration of hospital stay improved in about 40% of the studies. One or more markers of immune function and inflammatory responses were improved by ONS in 65% of the selected studies. These results suggest that appropriate use of ONS may be an ideal way to improve treatment efficacy; however, additional intervention trials are required to confirm these findings.
Keyword
MeSH Terms
Figure
Reference
-
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–86.
Article2. Santarpia L, Contaldo F, Pasanisi F. Nutritional screening and early treatment of malnutrition in cancer patients. J Cachexia Sarcopenia Muscle. 2011; 2:27–35.
Article3. Macciò A, Madeddu C, Gramignano G, Mulas C, Floris C, Sanna E, Cau MC, Panzone F, Mantovani G. A randomized phase III clinical trial of a combined treatment for cachexia in patients with gynecological cancers: evaluating the impact on metabolic and inflammatory profiles and quality of life. Gynecol Oncol. 2012; 124:417–425.
Article4. Van Cutsem E, Arends J. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs. 2005; 9:Suppl 2. S51–63.
Article5. Caccialanza R, Pedrazzoli P, Cereda E, Gavazzi C, Pinto C, Paccagnella A, Beretta GD, Nardi M, Laviano A, Zagonel V. Nutritional support in cancer patients: a position paper from the Italian Society of Medical Oncology (AIOM) and the Italian Society of Artificial Nutrition and Metabolism (SINPE). J Cancer. 2016; 7:131–135.
Article6. Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012; 16:153–166.
Article7. Melstrom LG, Melstrom KA Jr, Ding XZ, Adrian TE. Mechanisms of skeletal muscle degradation and its therapy in cancer cachexia. Histol Histopathol. 2007; 22:805–814.8. Palesty JA, Dudrick SJ. What we have learned about cachexia in gastrointestinal cancer. Dig Dis. 2003; 21:198–213.
Article9. Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, Biolo G, Boirie Y, Bosaeus I, Cederholm T, Costelli P, Fearon KC, Laviano A, Maggio M, Rossi Fanelli F, Schneider SM, Schols A, Sieber CC. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 2010; 29:154–159.
Article10. Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, Marks D, Mitch WE, Muscaritoli M, Najand A, Ponikowski P, Rossi Fanelli F, Schambelan M, Schols A, Schuster M, Thomas D, Wolfe R, Anker SD. Cachexia: a new definition. Clin Nutr. 2008; 27:793–799.
Article11. Johns N, Stephens NA, Fearon KC. Muscle wasting in cancer. Int J Biochem Cell Biol. 2013; 45:2215–2229.
Article12. Bozzetti F. Nutritional support of the oncology patient. Crit Rev Oncol Hematol. 2013; 87:172–200.
Article13. Nicolini A, Ferrari P, Masoni MC, Fini M, Pagani S, Giampietro O, Carpi A. Malnutrition, anorexia and cachexia in cancer patients: A mini-review on pathogenesis and treatment. Biomed Pharmacother. 2013; 67:807–817.
Article14. Argilés JM, Busquets S, Stemmler B, López-Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer. 2014; 14:754–762.
Article15. Petruzzelli M, Schweiger M, Schreiber R, Campos-Olivas R, Tsoli M, Allen J, Swarbrick M, Rose-John S, Rincon M, Robertson G, Zechner R, Wagner EF. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 2014; 20:433–447.
Article16. Kir S, White JP, Kleiner S, Kazak L, Cohen P, Baracos VE, Spiegelman BM. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014; 513:100–104.
Article17. Porporato PE. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis. 2016; 5:e200.
Article18. Kazi R, Kanagalingam J, Al-Mutairy A, Nutting CM, Clarke P, Rhys-Evans PH, Harrington KJ. Predictors of speech and swallowing function following primary surgery for oral and oropharyngeal cancer. Clin Otolaryngol. 2006; 31:83.
Article19. Haverkort EB, Binnekade JM, Busch OR, van Berge Henegouwen MI, de Haan RJ, Gouma DJ. Presence and persistence of nutrition-related symptoms during the first year following esophagectomy with gastric tube reconstruction in clinically disease-free patients. World J Surg. 2010; 34:2844–2852.
Article20. Ryan AM, Healy LA, Power DG, Rowley SP, Reynolds JV. Short-term nutritional implications of total gastrectomy for malignancy, and the impact of parenteral nutritional support. Clin Nutr. 2007; 26:718–727.
Article21. Bhosle J, Hall J. Principles of cancer treatment by chemotherapy. Surgery. 2006; 24:66–69.
Article22. Faria C, Li X, Nagl N, McBride A. Outcomes associated with 5-HT3-RA therapy selection in patients with chemotherapy-induced nausea and vomiting: a retrospective claims analysis. Am Health Drug Benefits. 2014; 7:50–58.23. Maranzano E, De Angelis V, Pergolizzi S, Lupattelli M, Frata P, Spagnesi S, Frisio ML, Mandoliti G, Malinverni G, Trippa F, Fabbietti L, Parisi S, Di Palma A, De Vecchi P, De Renzis C, Giorgetti C, Bergami T, Orecchia R, Portaluri M, Signor M, Di Gennaro D; Italian Group for Antiemetic Research in Radiotherapy - IGARR. A prospective observational trial on emesis in radiotherapy: analysis of 1020 patients recruited in 45 Italian radiation oncology centres. Radiother Oncol. 2010; 94:36–41.
Article24. Faber J, Uitdehaag MJ, Spaander M, van Steenbergen-Langeveld S, Vos P, Berkhout M, Lamers C, Rümke H, Tilanus H, Siersema P, van Helvoort A, van der Gaast A. Improved body weight and performance status and reduced serum PGE2 levels after nutritional intervention with a specific medical food in newly diagnosed patients with esophageal cancer or adenocarcinoma of the gastro-esophageal junction. J Cachexia Sarcopenia Muscle. 2015; 6:32–44.
Article25. Paccagnella A, Morello M, Da Mosto MC, Baruffi C, Marcon ML, Gava A, Baggio V, Lamon S, Babare R, Rosti G, Giometto M, Boscolo-Rizzo P, Kiwanuka E, Tessarin M, Caregaro L, Marchiori C. Early nutritional intervention improves treatment tolerance and outcomes in head and neck cancer patients undergoing concurrent chemoradiotherapy. Support Care Cancer. 2010; 18:837–845.
Article26. Trabal J, Leyes P, Forga M, Maurel J. Potential usefulness of an EPA-enriched nutritional supplement on chemotherapy tolerability in cancer patients without overt malnutrition. Nutr Hosp. 2010; 25:736–740.27. Vasson MP, Talvas J, Perche O, Dillies AF, Bachmann P, Pezet D, Achim AC, Pommier P, Racadot S, Weber A, Ramdani M, Kwiatkowski F, Bouteloup C. Immunonutrition improves functional capacities in head and neck and esophageal cancer patients undergoing radiochemotherapy: a randomized clinical trial. Clin Nutr. 2014; 33:204–210.
Article28. van der Meij BS, Langius JA, Spreeuwenberg MD, Slootmaker SM, Paul MA, Smit EF, van Leeuwen PA. Oral nutritional supplements containing n-3 polyunsaturated fatty acids affect quality of life and functional status in lung cancer patients during multimodality treatment: an RCT. Eur J Clin Nutr. 2012; 66:399–404.
Article29. Fietkau R, Lewitzki V, Kuhnt T, Hölscher T, Hess CF, Berger B, Wiegel T, Rödel C, Niewald M, Hermann RM, Lubgan D. A disease-specific enteral nutrition formula improves nutritional status and functional performance in patients with head and neck and esophageal cancer undergoing chemoradiotherapy: results of a randomized, controlled, multicenter trial. Cancer. 2013; 119:3343–3353.
Article30. Sunpaweravong S, Puttawibul P, Ruangsin S, Laohawiriyakamol S, Sunpaweravong P, Sangthawan D, Pradutkanchana J, Raungkhajorn P, Geater A. Randomized study of antiinflammatory and immune-modulatory effects of enteral immunonutrition during concurrent chemoradiotherapy for esophageal cancer. Nutr Cancer. 2014; 66:1–5.
Article31. Aiko S, Yoshizumi Y, Ishizuka T, Horio T, Sakano T, Kumano I, Kanai N, Maehara T. Enteral immuno-enhanced diets with arginine are safe and beneficial for patients early after esophageal cancer surgery. Dis Esophagus. 2008; 21:619–627.
Article32. De Luis DA, Izaola O, Cuellar L, Terroba MC, Martin T, Aller R. High dose of arginine enhanced enteral nutrition in postsurgical head and neck cancer patients. A randomized clinical trial. Eur Rev Med Pharmacol Sci. 2009; 13:279–283.33. de Luis DA, Aller R, Izaola O, Cuellar L, Terroba MC. Postsurgery enteral nutrition in head and neck cancer patients. Eur J Clin Nutr. 2002; 56:1126–1129.
Article34. Marano L, Porfidia R, Pezzella M, Grassia M, Petrillo M, Esposito G, Braccio B, Gallo P, Boccardi V, Cosenza A, Izzo G, Di Martino N. Clinical and immunological impact of early postoperative enteral immunonutrition after total gastrectomy in gastric cancer patients: a prospective randomized study. Ann Surg Oncol. 2013; 20:3912–3918.
Article35. Klek S, Sierzega M, Szybinski P, Szczepanek K, Scislo L, Walewska E, Kulig J. The immunomodulating enteral nutrition in malnourished surgical patients - a prospective, randomized, double-blind clinical trial. Clin Nutr. 2011; 30:282–288.
Article36. Buijs N, van Bokhorst-de van der Schueren MA, Langius JA, Leemans CR, Kuik DJ, Vermeulen MA, van Leeuwen PA. Perioperative arginine-supplemented nutrition in malnourished patients with head and neck cancer improves long-term survival. Am J Clin Nutr. 2010; 92:1151–1156.
Article37. Wang X, Pan L, Zhang P, Liu X, Wu G, Wang Y, Liu Y, Li N, Li J. Enteral nutrition improves clinical outcome and shortens hospital stay after cancer surgery. J Invest Surg. 2010; 23:309–313.
Article38. Trachootham D, Songkaew W, Hongsachum B, Wattana C, Changkluengdee N, Karapoch J, Thirdsuttironnapumi S, Meennuch E, Klaitong C, Sinthusek T, Lam-ubol A. Nutri-jelly may improve quality of life and decrease tube feeding demand in head and neck cancer patients. Support Care Cancer. 2015; 23:1421–1430.
Article39. Liumbruno GM, Bennardello F, Lattanzio A, Piccoli P, Rossettias G; Italian Society of Transfusion Medicine and Immunohaematology (SIMTI). Recommendations for the use of albumin and immunoglobulins. Blood Transfus. 2009; 7:216–234.40. Pupim LB, Cuppari L, Ikizler TA. Nutrition and metabolism in kidney disease. Semin Nephrol. 2006; 26:134–157.
Article41. Banh L. Serum proteins as markers of nutrition: what are we treating? Pract Gastroenterol. 2006; 30:46–64.42. Friedman AN, Fadem SZ. Reassessment of albumin as a nutritional marker in kidney disease. J Am Soc Nephrol. 2010; 21:223–230.
Article43. Aoyama T, Yoshikawa T, Shirai J, Hayashi T, Yamada T, Tsuchida K, Hasegawa S, Cho H, Yukawa N, Oshima T, Rino Y, Masuda M, Tsuburaya A. Body weight loss after surgery is an independent risk factor for continuation of S-1 adjuvant chemotherapy for gastric cancer. Ann Surg Oncol. 2013; 20:2000–2006.
Article44. Chawda JG, Jain SS, Patel HR, Chaduvula N, Patel K. The relationship between serum lipid levels and the risk of oral cancer. Indian J Med Paediatr Oncol. 2011; 32:34–37.
Article45. Rauchhaus M, Coats AJ, Anker SD. The endotoxin-lipoprotein hypothesis. Lancet. 2000; 356:930–933.
Article46. Vigano AL, di Tomasso J, Kilgour RD, Trutschnigg B, Lucar E, Morais JA, Borod M. The abridged patient-generated subjective global assessment is a useful tool for early detection and characterization of cancer cachexia. J Acad Nutr Diet. 2014; 114:1088–1098.
Article47. Boléo-Tomé C, Monteiro-Grillo I, Camilo M, Ravasco P. Validation of the malnutrition universal screening tool (MUST) in cancer. Br J Nutr. 2012; 108:343–348.
Article48. Galizia G, Orditura M, Romano C, Lieto E, Castellano P, Pelosio L, Imperatore V, Catalano G, Pignatelli C, De Vita F. Prognostic significance of circulating IL-10 and IL-6 serum levels in colon cancer patients undergoing surgery. Clin Immunol. 2002; 102:169–178.
Article49. Lopes CO, Callera F. Three-dimensional conformal radiotherapy in prostate cancer patients: rise in interleukin 6 (IL-6) but not IL-2, IL-4, IL-5, tumor necrosis factor-α, MIP-1-α, and LIF levels. Int J Radiat Oncol Biol Phys. 2012; 82:1385–1388.
Article50. Baldwin C, Spiro A, Ahern R, Emery PW. Oral nutritional interventions in malnourished patients with cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2012; 104:371–385.
Article51. Cocks K, King MT, Velikova G, de Castro G Jr, Martyn St-James M, Fayers PM, Brown JM. Evidence-based guidelines for interpreting change scores for the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. Eur J Cancer. 2012; 48:1713–1721.
Article52. Calder PC. Rationale and use of n-3 fatty acids in artificial nutrition. Proc Nutr Soc. 2010; 69:565–573.
Article53. Fenton JI, Hord NG, Ghosh S, Gurzell EA. Immunomodulation by dietary long chain omega-3 fatty acids and the potential for adverse health outcomes. Prostaglandins Leukot Essent Fatty Acids. 2013; 89:379–390.
Article54. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002; 420:860–867.
Article55. Argiles JM, Lopez-Soriano FJ, Busquets S. Counteracting inflammation: a promising therapy in cachexia. Crit Rev Oncog. 2012; 17:253–262.
Article56. Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004; 361:184–187.
Article57. Graziano F, Ruzzo A, Santini D, Humar B, Tonini G, Catalano V, Berardi R, Pizzagalli F, Arduini F, Bearzi I, Scartozzi M, Cascinu S, Testa E, Ficarelli R, Magnani M. Prognostic role of interleukin-1beta gene and interleukin-1 receptor antagonist gene polymorphisms in patients with advanced gastric cancer. J Clin Oncol. 2005; 23:2339–2345.
Article58. Kuroda K, Nakashima J, Kanao K, Kikuchi E, Miyajima A, Horiguchi Y, Nakagawa K, Oya M, Ohigashi T, Murai M. Interleukin 6 is associated with cachexia in patients with prostate cancer. Urology. 2007; 69:113–117.
Article59. Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006; 83:Suppl. 1505S–19S.
Article60. Vedin I, Cederholm T, Freund Levi Y, Basun H, Garlind A, Faxén Irving G, Jönhagen ME, Vessby B, Wahlund LO, Palmblad J. Effects of docosahexaenoic acid-rich n-3 fatty acid supplementation on cytokine release from blood mononuclear leukocytes: the OmegAD study. Am J Clin Nutr. 2008; 87:1616–1622.
Article61. Han SN, Lichtenstein AH, Ausman LM, Meydani SN. Novel soybean oils differing in fatty acid composition alter immune functions of moderately hypercholesterolemic older adults. J Nutr. 2012; 142:2182–2187.
Article62. Calder PC. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015; 1851:469–484.
Article63. Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005; 5:641–654.
Article64. Popovic PJ, Zeh HJ 3rd, Ochoa JB. Arginine and immunity. J Nutr. 2007; 137:Suppl 2. 1681S–6S.
Article65. Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O’Neill A, Mier J, Ochoa AC. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005; 65:3044–3048.
Article66. Werner A, Amann E, Schnitzius V, Habermeier A, Luckner-Minden C, Leuchtner N, Rupp J, Closs EI, Munder M. Induced arginine transport via cationic amino acid transporter-1 is necessary for human T-cell proliferation. Eur J Immunol. 2016; 46:92–103.
Article67. Sakai T, Taki T, Nakamoto A, Tazaki S, Arakawa M, Nakamoto M, Tsutsumi R, Shuto E. Dietary ribonucleic acid suppresses inflammation of adipose tissue and improves glucose intolerance that is mediated by immune cells in C57BL/6 mice fed a high-fat diet. J Nutr Sci Vitaminol (Tokyo). 2015; 61:73–78.
Article68. Nagafuchi S, Hachimura S, Totsuka M, Takahashi T, Goto M, Yajima T, Kuwata T, Habu S, Kaminogawa S. Dietary nucleotides can up-regulate antigen-specific Th1 immune responses and suppress antigen-specific IgE responses in mice. Int Arch Allergy Immunol. 2000; 122:33–41.
Article69. Jyonouchi H, Sun S, Winship T, Kuchan MJ. Dietary ribonucleotides increase antigen-specific type 1 T-helper cells in the regional draining lymph nodes in young BALB/cJ mice. Nutrition. 2003; 19:41–46.
Article70. Sudo N, Aiba Y, Takaki A, Tanaka K, Yu XN, Oyama N, Koga Y, Kubo C. Dietary nucleic acids promote a shift in Th1/Th2 balance toward Th1-dominant immunity. Clin Exp Allergy. 2000; 30:979–987.
Article71. Tomiya T, Omata M, Fujiwara K. Significance of branched chain amino acids as possible stimulators of hepatocyte growth factor. Biochem Biophys Res Commun. 2004; 313:411–416.
Article72. Tajiri K, Shimizu Y. Branched-chain amino acids in liver diseases. World J Gastroenterol. 2013; 19:7620–7629.
Article73. Newsholme P. Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J Nutr. 2001; 131:Suppl. 2515S–22S.74. Gottfried E, Kreutz M, Mackensen A. Tumor metabolism as modulator of immune response and tumor progression. Semin Cancer Biol. 2012; 22:335–341.
Article75. Sikalidis AK. Amino acids and immune response: a role for cysteine, glutamine, phenylalanine, tryptophan and arginine in T-cell function and cancer? Pathol Oncol Res. 2015; 21:9–17.
Article76. Melis GC, Boelens PG, van der Sijp JR, Popovici T, De Bandt JP, Cynober L, van Leeuwen PA. The feeding route (enteral or parenteral) affects the plasma response of the dipetide Ala-Gln and the amino acids glutamine, citrulline and arginine, with the administration of Ala-Gln in preoperative patients. Br J Nutr. 2005; 94:19–26.
Article77. Diestel CF, Marques RG, Lopes-Paulo F, Paiva D, Horst NL, Caetano CE, Portela MC. Role of L-glutamine and glycine supplementation on irradiated colonic wall. Int J Colorectal Dis. 2007; 22:1523–1529.
Article78. Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, Fearon K, Hütterer E, Isenring E, Kaasa S, Krznaric Z, Laird B, Larsson M, Laviano A, Mühlebach S, Muscaritoli M, Oldervoll L, Ravasco P, Solheim T, Strasser F, de van der Schueren M, Preiser JC. de van der Schueren M, Preiser JC.ESPEN guidelines on nutrition in cancer patients. Clin Nutr. Forthcoming. 2016.79. Okabayashi T, Iyoki M, Sugimoto T, Kobayashi M, Hanazaki K. Oral supplementation with carbohydrate- and branched-chain amino acid-enriched nutrients improves postoperative quality of life in patients undergoing hepatic resection. Amino Acids. 2011; 40:1213–1220.
Article80. Turnock A, Calder PC, West AL, Izzard M, Morton RP, Plank LD. Perioperative immunonutrition in well-nourished patients undergoing surgery for head and neck cancer: evaluation of inflammatory and immunologic outcomes. Nutrients. 2013; 5:1186–1199.
Article81. Felekis D, Eleftheriadou A, Papadakos G, Bosinakou I, Ferekidou E, Kandiloros D, Katsaragakis S, Charalabopoulos K, Manolopoulos L. Effect of perioperative immuno-enhanced enteral nutrition on inflammatory response, nutritional status, and outcomes in head and neck cancer patients undergoing major surgery. Nutr Cancer. 2010; 62:1105–1112.
Article82. McGough C, Wedlake L, Baldwin C, Hackett C, Norman AR, Blake P, Harrington K, Tait D, Khoo V, Frost G, Andreyev HJ. Clinical trial: normal diet vs. partial replacement with oral E028 formula for the prevention of gastrointestinal toxicity in cancer patients undergoing pelvic radiotherapy. Aliment Pharmacol Ther. 2008; 27:1132–1139.
Article83. Faber J, Berkhout M, Fiedler U, Avlar M, Witteman BJ, Vos AP, Henke M, Garssen J, van Helvoort A, Otten MH, Arends J. Rapid EPA and DHA incorporation and reduced PGE2 levels after one week intervention with a medical food in cancer patients receiving radiotherapy, a randomized trial. Clin Nutr. 2013; 32:338–345.
Article84. Kuroda H, Ushio A, Miyamoto Y, Sawara K, Oikawa K, Kasai K, Endo R, Takikawa Y, Kato A, Suzuki K. Effects of branched-chain amino acid-enriched nutrient for patients with hepatocellular carcinoma following radiofrequency ablation: a one-year prospective trial. J Gastroenterol Hepatol. 2010; 25:1550–1555.
Article85. Sorensen D, McCarthy M, Baumgartner B, Demars S. Perioperative immunonutrition in head and neck cancer. Laryngoscope. 2009; 119:1358–1364.
Article86. Deutz NE, Safar A, Schutzler S, Memelink R, Ferrando A, Spencer H, van Helvoort A, Wolfe RR. Muscle protein synthesis in cancer patients can be stimulated with a specially formulated medical food. Clin Nutr. 2011; 30:759–768.
Article87. van der Meij BS, Langius JA, Smit EF, Spreeuwenberg MD, von Blomberg BM, Heijboer AC, Paul MA, van Leeuwen PA. Oral nutritional supplements containing (n-3) polyunsaturated fatty acids affect the nutritional status of patients with stage III non-small cell lung cancer during multimodality treatment. J Nutr. 2010; 140:1774–1780.
Article88. Fujitani K, Tsujinaka T, Fujita J, Miyashiro I, Imamura H, Kimura Y, Kobayashi K, Kurokawa Y, Shimokawa T, Furukawa H; Osaka Gastrointestinal Cancer Chemotherapy Study Group. Prospective randomized trial of preoperative enteral immunonutrition followed by elective total gastrectomy for gastric cancer. Br J Surg. 2012; 99:621–629.
Article89. de Luis DA, Izaola O, Cuellar L, Terroba MC, Arranz M, Fernandez N, Aller R. Effect of c-reactive protein and interleukins blood levels in postsurgery arginine-enhanced enteral nutrition in head and neck cancer patients. Eur J Clin Nutr. 2003; 57:96–99.
Article90. Klek S, Kulig J, Sierzega M, Szczepanek K, Szybiński P, Scislo L, Walewska E, Kubisz A, Szczepanik AM. Standard and immunomodulating enteral nutrition in patients after extended gastrointestinal surgery--a prospective, randomized, controlled clinical trial. Clin Nutr. 2008; 27:504–512.
Article91. Ryan AM, Reynolds JV, Healy L, Byrne M, Moore J, Brannelly N, McHugh A, McCormack D, Flood P. Enteral nutrition enriched with eicosapentaenoic acid (EPA) preserves lean body mass following esophageal cancer surgery: results of a double-blinded randomized controlled trial. Ann Surg. 2009; 249:355–363.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nutritional Care of Gastric Cancer Patients with Clinical Outcomes and Complications: A Review
- Nutritional Intervention Using Nutrition Care Process in a Malnourished Patient with Chemotherapy Side Effects
- Use of Oral Nutritional Supplements for Patients with Diabetes
- Analysis of the Factors Relating Nutritional Status in Discharging of Leukemia Patients Receiving Chemotherapy
- Evaluation on the Time to Start Parenteral Nutrition in Hospitalized Cancer Patients